Dissociable regulation of instrumental action within mouse prefrontal cortex
- PMID: 21044173
- PMCID: PMC3058746
- DOI: 10.1111/j.1460-9568.2010.07438.x
Dissociable regulation of instrumental action within mouse prefrontal cortex
Abstract
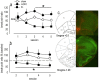
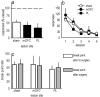
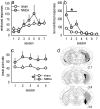
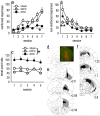
Evaluation of the behavioral 'costs', such as effort expenditure relative to the benefits of obtaining reward, is a major determinant of goal-directed action. Neuroimaging evidence suggests that the human medial orbitofrontal cortex (mOFC) is involved in this calculation and thereby guides goal-directed and choice behavior, but this region's functional significance in rodents is unknown despite extensive work characterizing the role of the lateral OFC in cue-related response inhibition processes. We first tested mice with mOFC lesions in an instrumental reversal task lacking discrete cues signaling reinforcement; here, animals were required to shift responding based on the location of the reinforced aperture within the chamber. Mice with mOFC lesions acquired the reversal but failed to inhibit responding on the previously reinforced aperture, while mice with prelimbic prefrontal cortex lesions were unaffected. When tested on a progressive ratio schedule of reinforcement, mice with prelimbic cortical lesions were unable to maintain responding, resulting in declining response levels. Mice with mOFC lesions, by contrast, escalated responding. Neither lesion affected sensitivity to satiety-specific outcome devaluation or non-reinforcement (i.e. extinction), and neither had effects when placed after animals were trained on a progressive ratio response schedule. Lesions of the ventral hippocampus, which projects to the mOFC, resulted in similar response patterns, while lateral OFC and dorsal hippocampus lesions resulted in response acquisition, though not inhibition, deficits in an instrumental reversal. Our findings thus selectively implicate the rodent mOFC in braking reinforced goal-directed action when reinforcement requires the acquisition of novel response contingencies.
© 2010 The Authors. European Journal of Neuroscience © 2010 Federation of European Neuroscience Societies and Blackwell Publishing Ltd.
Figures

Similar articles
-
The Medial Orbitofrontal Cortex Regulates Sensitivity to Outcome Value.J Neurosci. 2016 Apr 20;36(16):4600-13. doi: 10.1523/JNEUROSCI.4253-15.2016. J Neurosci. 2016. PMID: 27098701 Free PMC article.
-
Inactivation of the infralimbic prefrontal cortex reinstates goal-directed responding in overtrained rats.Behav Brain Res. 2003 Nov 30;146(1-2):167-74. doi: 10.1016/j.bbr.2003.09.025. Behav Brain Res. 2003. PMID: 14643469
-
Inferring action-dependent outcome representations depends on anterior but not posterior medial orbitofrontal cortex.Neurobiol Learn Mem. 2018 Nov;155:463-473. doi: 10.1016/j.nlm.2018.09.008. Epub 2018 Sep 19. Neurobiol Learn Mem. 2018. PMID: 30243849
-
Involvement of the rodent prelimbic and medial orbitofrontal cortices in goal-directed action: A brief review.J Neurosci Res. 2020 Jun;98(6):1020-1030. doi: 10.1002/jnr.24567. Epub 2019 Dec 10. J Neurosci Res. 2020. PMID: 31820488 Free PMC article. Review.
-
New functions of the rodent prelimbic and infralimbic cortex in instrumental behavior.Neurobiol Learn Mem. 2021 Nov;185:107533. doi: 10.1016/j.nlm.2021.107533. Epub 2021 Oct 18. Neurobiol Learn Mem. 2021. PMID: 34673264 Free PMC article. Review.
Cited by
-
Input- and Output-Specific Regulation of Serial Order Performance by Corticostriatal Circuits.Neuron. 2015 Oct 21;88(2):345-56. doi: 10.1016/j.neuron.2015.09.035. Neuron. 2015. PMID: 26494279 Free PMC article.
-
Prefrontal cortical BDNF: A regulatory key in cocaine- and food-reinforced behaviors.Neurobiol Dis. 2016 Jul;91:326-35. doi: 10.1016/j.nbd.2016.02.021. Epub 2016 Feb 26. Neurobiol Dis. 2016. PMID: 26923993 Free PMC article. Review.
-
β1-Integrins in the Developing Orbitofrontal Cortex Are Necessary for Expectancy Updating in Mice.J Neurosci. 2019 Aug 21;39(34):6644-6655. doi: 10.1523/JNEUROSCI.3072-18.2019. Epub 2019 Jun 28. J Neurosci. 2019. PMID: 31253753 Free PMC article.
-
Impulsive Action and Impulsive Choice Are Differentially Associated With Gene Expression Variations of the GABAA Receptor Alfa 1 Subunit and the CB1 Receptor in the Lateral and Medial Orbitofrontal Cortices.Front Behav Neurosci. 2019 Feb 20;13:22. doi: 10.3389/fnbeh.2019.00022. eCollection 2019. Front Behav Neurosci. 2019. PMID: 30842730 Free PMC article.
-
Parallel declines in cognition, motivation, and locomotion in aging mice: association with immune gene upregulation in the medial prefrontal cortex.Exp Gerontol. 2011 Aug;46(8):643-59. doi: 10.1016/j.exger.2011.03.003. Epub 2011 Mar 29. Exp Gerontol. 2011. PMID: 21453768 Free PMC article.
References
-
- Baldwin AE, Holahan MR, Sadeghian K, Kelley AE. N-methyl-D-aspartate receptor-dependent plasticity within a distributed corticostriatal network mediates appetitive instrumental learning. Behav Neurosci. 2000;114:84–98. - PubMed
-
- Baldwin AE, Sadeghian K, Holahan MR, Kelley AE. Appetitive instrumental learning is impaired by inhibition of cAMP-dependent protein kinase within the nucleus accumbens. Neurobiol Learn Mem. 2002;77:44–62. - PubMed
-
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 DA015222/DA/NIDA NIH HHS/United States
- AA017537/AA/NIAAA NIH HHS/United States
- K08 MH081190/MH/NIMH NIH HHS/United States
- P01 MH025642/MH/NIMH NIH HHS/United States
- F31 MH079680/MH/NIMH NIH HHS/United States
- UL1 DE019586/DE/NIDCR NIH HHS/United States
- RL1 AA017537/AA/NIAAA NIH HHS/United States
- R01 DA011717/DA/NIDA NIH HHS/United States
- MH025642/MH/NIMH NIH HHS/United States
- MH066172/MH/NIMH NIH HHS/United States
- DA011717/DA/NIDA NIH HHS/United States
- P50 MH066172/MH/NIMH NIH HHS/United States
- UL1-DE19586/DE/NIDCR NIH HHS/United States
- MH079680/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources

