Post-traumatic seizure susceptibility is attenuated by hypothermia therapy
- PMID: 21044182
- PMCID: PMC3059103
- DOI: 10.1111/j.1460-9568.2010.07467.x
Post-traumatic seizure susceptibility is attenuated by hypothermia therapy
Abstract
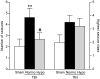
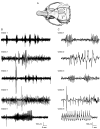
Traumatic brain injury (TBI) is a major risk factor for the subsequent development of epilepsy. Currently, chronic seizures after brain injury are often poorly controlled by available antiepileptic drugs. Hypothermia treatment, a modest reduction in brain temperature, reduces inflammation, activates pro-survival signaling pathways, and improves cognitive outcome after TBI. Given the well-known effect of therapeutic hypothermia to ameliorate pathological changes in the brain after TBI, we hypothesized that hypothermia therapy may attenuate the development of post-traumatic epilepsy and some of the pathomechanisms that underlie seizure formation. To test this hypothesis, adult male Sprague Dawley rats received moderate parasagittal fluid-percussion brain injury, and were then maintained at normothermic or moderate hypothermic temperatures for 4 h. At 12 weeks after recovery, seizure susceptibility was assessed by challenging the animals with pentylenetetrazole, a GABA(A) receptor antagonist. Pentylenetetrazole elicited a significant increase in seizure frequency in TBI normothermic animals as compared with sham surgery animals and this was significantly reduced in TBI hypothermic animals. Early hypothermia treatment did not rescue chronic dentate hilar neuronal loss nor did it improve loss of doublecortin-labeled cells in the dentate gyrus post-seizures. However, mossy fiber sprouting was significantly attenuated by hypothermia therapy. These findings demonstrate that reductions in seizure susceptibility after TBI are improved with post-traumatic hypothermia and provide a new therapeutic avenue for the treatment of post-traumatic epilepsy.
© 2010 The Authors. European Journal of Neuroscience © 2010 Federation of European Neuroscience Societies and Blackwell Publishing Ltd.
Figures



References
-
- Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978;182:851–914. - PubMed
-
- Andre V, Pineau N, Motte JE, Marescaux C, Nehlig A. Mapping of neuronal networks underlying generalized seizures induced by increasing doses of pentylenetetrazol in the immature and adult rat: a c-Fos immunohistochemical study. Eur J Neurosci. 1998;10:2094–2106. - PubMed
-
- Annegers JF, Hauser WA, Coan SP, Rocca WA. A population-based study of seizures after traumatic brain injuries. N Engl J Med. 1998;338:20–24. - PubMed
-
- Atkins CM, Oliva AA, Jr, Alonso OF, Chen S, Bramlett HM, Hu BR, Dietrich WD. Hypothermia treatment potentiates ERK1/2 activation after traumatic brain injury. Eur J Neurosci. 2007a;26:810–819. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

