In vivo development of dendritic orientation in wild-type and mislocalized retinal ganglion cells
- PMID: 21044295
- PMCID: PMC2988773
- DOI: 10.1186/1749-8104-5-29
In vivo development of dendritic orientation in wild-type and mislocalized retinal ganglion cells
Abstract
Background: Many neurons in the central nervous system, including retinal ganglion cells (RGCs), possess asymmetric dendritic arbors oriented toward their presynaptic partners. How such dendritic arbors become biased during development in vivo is not well understood. Dendritic arbors may become oriented by directed outgrowth or by reorganization of an initially unbiased arbor. To distinguish between these possibilities, we imaged the dynamic behavior of zebrafish RGC dendrites during development in vivo. We then addressed how cell positioning within the retina, altered in heart-and-soul (has) mutants, affects RGC dendritic orientation.
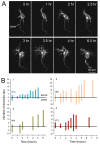
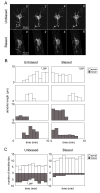
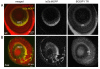
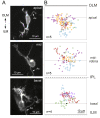
Results: In vivo multiphoton time-lapse analysis revealed that RGC dendrites initially exhibit exploratory behavior in multiple directions but progressively become apically oriented. The lifetimes of basal and apical dendrites were generally comparable before and during the period when arbors became biased. However, with maturation, the addition and extension rates of basal dendrites were slower than those of the apical dendrites. Oriented dendritic arbors were also found in misplaced RGCs of the has retina but there was no preferred orientation amongst the population. However, has RGCs always projected dendrites toward nearby neuropil where amacrine and bipolar cell neurites also terminated. Chimera analysis showed that the abnormal dendritic organization of RGCs in the mutant was non-cell autonomous.
Conclusions: Our observations show that RGC dendritic arbors acquire an apical orientation by selective and gradual restriction of dendrite addition to the apical side of the cell body, rather than by preferential dendrite stabilization or elimination. A biased arbor emerges at a stage when many of the dendritic processes still appear exploratory. The generation of an oriented RGC dendritic arbor is likely to be determined by cell-extrinsic cues. Such cues are unlikely to be localized to the basal lamina of the inner retina, but rather may be provided by cells presynaptic to the RGCs.
Figures




Similar articles
-
In vivo imaging reveals dendritic targeting of laminated afferents by zebrafish retinal ganglion cells.Neuron. 2006 Nov 22;52(4):609-21. doi: 10.1016/j.neuron.2006.10.004. Neuron. 2006. PMID: 17114046 Free PMC article.
-
Cellular and Molecular Analysis of Dendritic Morphogenesis in a Retinal Cell Type That Senses Color Contrast and Ventral Motion.J Neurosci. 2017 Dec 13;37(50):12247-12262. doi: 10.1523/JNEUROSCI.2098-17.2017. Epub 2017 Nov 7. J Neurosci. 2017. PMID: 29114073 Free PMC article.
-
Factors controlling the dendritic arborization of retinal ganglion cells.Vis Neurosci. 1996 Jul-Aug;13(4):721-33. doi: 10.1017/s0952523800008609. Vis Neurosci. 1996. PMID: 8870228
-
Retinal ganglion cell dendritic development and its control. Filling the gaps.Mol Neurobiol. 1996 Apr;12(2):133-44. doi: 10.1007/BF02740650. Mol Neurobiol. 1996. PMID: 8818147 Review.
-
Pathway-specific maturation, visual deprivation, and development of retinal pathway.Neuroscientist. 2004 Aug;10(4):337-46. doi: 10.1177/1073858404265254. Neuroscientist. 2004. PMID: 15271261 Review.
Cited by
-
Inhibitory neuron migration and IPL formation in the developing zebrafish retina.Development. 2015 Aug 1;142(15):2665-77. doi: 10.1242/dev.122473. Epub 2015 Jun 26. Development. 2015. PMID: 26116662 Free PMC article.
-
HuC-eGFP mosaic labelling of neurons in zebrafish enables in vivo live cell imaging of growth cones.J Mol Histol. 2012 Dec;43(6):615-23. doi: 10.1007/s10735-012-9462-7. Epub 2012 Oct 27. J Mol Histol. 2012. PMID: 23104578
-
An Antagonistic Axon-Dendrite Interplay Enables Efficient Neuronal Repair in the Adult Zebrafish Central Nervous System.Mol Neurobiol. 2019 May;56(5):3175-3192. doi: 10.1007/s12035-018-1292-5. Epub 2018 Aug 13. Mol Neurobiol. 2019. PMID: 30105671
-
Live imaging and analysis of postnatal mouse retinal development.BMC Dev Biol. 2013 Jun 10;13:24. doi: 10.1186/1471-213X-13-24. BMC Dev Biol. 2013. PMID: 23758927 Free PMC article.
-
Opportunities and challenges for using the zebrafish to study neuronal connectivity as an endpoint of developmental neurotoxicity.Neurotoxicology. 2018 Jul;67:102-111. doi: 10.1016/j.neuro.2018.04.016. Epub 2018 Apr 25. Neurotoxicology. 2018. PMID: 29704525 Free PMC article. Review.
References
-
- Ramon y Cajal S. Studies on Vertebrate Neurogenesis. Springfield, IL: Thomas; 1960.
-
- Ramon y Cajal S. The Structure of the Retina. Springfield, IL: CC Thomas; 1972.
-
- Greenough WT, Chang FL. Dendritic pattern formation involves both oriented regression and oriented growth in the barrels of mouse somatosensory cortex. Brain Res. 1988;471:148–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

