Increasing CD44+/CD24(-) tumor stem cells, and upregulation of COX-2 and HDAC6, as major functions of HER2 in breast tumorigenesis
- PMID: 21044318
- PMCID: PMC2989327
- DOI: 10.1186/1476-4598-9-288
Increasing CD44+/CD24(-) tumor stem cells, and upregulation of COX-2 and HDAC6, as major functions of HER2 in breast tumorigenesis
Abstract
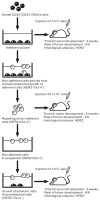
Background: Cancer cells are believed to arise primarily from stem cells. CD44+/CD24(-) have been identified as markers for human breast cancer stem cells. Although, HER2 is a well known breast cancer oncogene, the mechanisms of action of this gene are not completely understood. Previously, we have derived immortal (M13SV1), weakly tumorigenic (M13SV1R2) and highly tumorigenic (M13SV1R2N1) cell lines from a breast epithelial cell type with stem cell phenotypes after successive SV40 large T-antigen transfection, X-ray irradiation and ectopic expression of HER2/C-erbB2/neu. Recently, we found that M13SV1R2 cells became non-tumorigenic after growing in a growth factor/hormone-deprived medium (R2d cells).
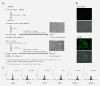
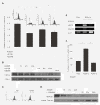
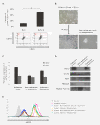
Results: In this study, we developed M13SV1R2N1 under the same growth factor/hormone-deprived condition (R2N1d cells). This provides an opportunity to analyze HER2 effect on gene expression associated with tumorigenesis by comparative study of R2d and R2N1d cells with homogeneous genetic background except HER2 expression. The results reveal distinct characters of R2N1d cells that can be ascribed to HER2: 1) development of fast-growing tumors; 2) high frequency of CD44+/CD24(-) cells (~50% for R2N1d vs. ~10% for R2d); 3) enhanced expression of COX-2, HDAC6 mediated, respectively, by MAPK and PI3K/Akt pathways, and many genes associated with inflammation, metastasis, and angiogenesis. Furthermore, HER2 expression can be down regulated in non-adhering R2N1d cells. These cells showed longer latent period and lower rate of tumor development compared with adhering cells.
Conclusions: HER2 may induce breast cancer by increasing the frequency of tumor stem cells and upregulating the expression of COX-2 and HDAC6 that play pivotal roles in tumor progression.
Figures



Similar articles
-
HER2-associated radioresistance of breast cancer stem cells isolated from HER2-negative breast cancer cells.Clin Cancer Res. 2012 Dec 15;18(24):6634-47. doi: 10.1158/1078-0432.CCR-12-1436. Epub 2012 Oct 22. Clin Cancer Res. 2012. PMID: 23091114 Free PMC article.
-
Modulation of tumorigenesis and oestrogen receptor-alpha expression by cell culture conditions in a stem cell-derived breast epithelial cell line.Biol Cell. 2010 Jan 6;102(3):159-72. doi: 10.1042/BC20090132. Biol Cell. 2010. PMID: 19895368
-
Expression of CD24 is associated with HER2 expression and supports HER2-Akt signaling in HER2-positive breast cancer cells.Cancer Sci. 2014 Jul;105(7):779-87. doi: 10.1111/cas.12427. Epub 2014 Jun 2. Cancer Sci. 2014. PMID: 24754246 Free PMC article.
-
Breast cancer stem cells and intrinsic subtypes: controversies rage on.Curr Stem Cell Res Ther. 2009 Jan;4(1):50-60. doi: 10.2174/157488809787169110. Curr Stem Cell Res Ther. 2009. PMID: 19149630 Review.
-
Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44(hi/)CD24 (lo/-) stem cell phenotype in human breast cancer.J Mammary Gland Biol Neoplasia. 2010 Jun;15(2):235-52. doi: 10.1007/s10911-010-9175-z. Epub 2010 Jun 4. J Mammary Gland Biol Neoplasia. 2010. PMID: 20521089 Review.
Cited by
-
Isoflurane promotes proliferation of squamous cervical cancer cells through mTOR-histone deacetylase 6 pathway.Mol Cell Biochem. 2021 Jan;476(1):45-55. doi: 10.1007/s11010-020-03884-7. Epub 2020 Aug 24. Mol Cell Biochem. 2021. PMID: 32833118 Free PMC article.
-
Cancer stem cell markers ALDH1 and CD44+/CD24- phenotype and their prognosis impact in invasive ductal carcinoma.Eur J Histochem. 2018 Sep 3;62(3):2943. doi: 10.4081/ejh.2018.2943. Eur J Histochem. 2018. PMID: 30362671 Free PMC article.
-
Cyclooxygenase-2: A Role in Cancer Stem Cell Survival and Repopulation of Cancer Cells during Therapy.Stem Cells Int. 2016;2016:2048731. doi: 10.1155/2016/2048731. Epub 2016 Nov 1. Stem Cells Int. 2016. PMID: 27882058 Free PMC article. Review.
-
Changes of Mutations and Copy-Number and Enhanced Cell Migration during Breast Tumorigenesis.Adv Biol (Weinh). 2023 Feb;7(2):e2200072. doi: 10.1002/adbi.202200072. Epub 2022 Nov 30. Adv Biol (Weinh). 2023. PMID: 36449747 Free PMC article.
-
Lifespan Extension and Sustained Expression of Stem Cell Phenotype of Human Breast Epithelial Stem Cells in a Medium with Antioxidants.Stem Cells Int. 2016;2016:4591310. doi: 10.1155/2016/4591310. Epub 2016 Oct 11. Stem Cells Int. 2016. PMID: 27807451 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

