The role of thin filament cooperativity in cardiac length-dependent calcium activation
- PMID: 21044595
- PMCID: PMC2965940
- DOI: 10.1016/j.bpj.2010.09.003
The role of thin filament cooperativity in cardiac length-dependent calcium activation
Abstract
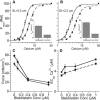
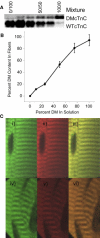
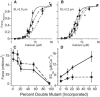
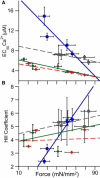
Length-dependent activation (LDA) is a prominent feature of cardiac muscle characterized by decreases in the Ca(2+) levels required to generate force (i.e., increases in Ca(2+) sensitivity) when muscle is stretched. Previous studies have concluded that LDA originates from the increased ability of (strong) cross-bridges to attach when muscle is lengthened, which in turn enhances Ca(2+) binding to the troponin C (TnC) subunit of the troponin complex. However, our results demonstrate that inhibition of strong cross-bridge attachment with blebbistatin had no effect on the length-dependent modulation of Ca(2+) sensitivity (i.e., EC(50)) or Ca(2+) cooperativity, suggesting that LDA originates upstream of cross-bridge attachment. To test whether LDA arises from length dependence of thin-filament activation, we replaced native cTnC with a mutant cTnC (DM-TnC) that is incapable of binding Ca(2+). Although progressive replacement of native cTnC with DM-TnC caused an expected monotonic decrease in the maximal force (F(max)), DM-TnC incorporation induced much larger increases in EC(50) and decreases in Ca(2+) cooperativity at short lengths than at long lengths. These findings support the conclusion that LDA arises primarily from the influence of length on the modulation of the Ca(2+) cooperativity arising from interaction between adjacent troponin-tropomyosin complexes on the thin filament.
Copyright © 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Allen D.G., Kentish J.C. The cellular basis of the length-tension relation in cardiac muscle. J. Mol. Cell. Cardiol. 1985;17:821–840. - PubMed
-
- Dobesh D.P., Konhilas J.P., de Tombe P.P. Cooperative activation in cardiac muscle: impact of sarcomere length. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H1055–H1062. - PubMed
-
- Gordon A.M., Homsher E., Regnier M. Regulation of contraction in striated muscle. Physiol. Rev. 2000;80:853–924. - PubMed
-
- Kentish J.C., ter Keurs H.E., Noble M.I. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circ. Res. 1986;58:755–768. - PubMed
-
- de Beer E.L., Grundeman R.L., Schiereck P. Effect of sarcomere length and filament lattice spacing on force development in skinned cardiac and skeletal muscle preparations from the rabbit. Basic Res. Cardiol. 1988;83:410–423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

