Single molecule characterization of α-synuclein in aggregation-prone states
- PMID: 21044603
- PMCID: PMC2965999
- DOI: 10.1016/j.bpj.2010.08.056
Single molecule characterization of α-synuclein in aggregation-prone states
Abstract
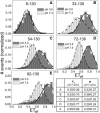
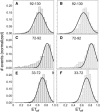
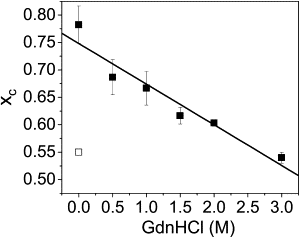
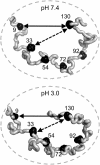
α-Synuclein (αS) is an intrinsically disordered protein whose aggregation into ordered, fibrillar structures underlies the pathogenesis of Parkinson's disease. A full understanding of the factors that cause its conversion from soluble protein to insoluble aggregate requires characterization of the conformations of the monomer protein under conditions that favor aggregation. Here we use single molecule Förster resonance energy transfer to probe the structure of several aggregation-prone states of αS. Both low pH and charged molecules have been shown to accelerate the aggregation of αS and induce conformational changes in the protein. We find that at low pH, the C-terminus of αS undergoes substantial collapse, with minimal effect on the N-terminus and central region. The proximity of the N- and C-termini and the global dimensions of the protein are relatively unaffected by the C-terminal collapse. Moreover, although compact at low pH, with restricted chain motion, the structure of the C-terminus appears to be random. Low pH has a dramatically different effect on αS structure than the molecular aggregation inducers spermine and heparin. Binding of these molecules gives rise to only minor conformational changes in αS, suggesting that their mechanism of aggregation enhancement is fundamentally different from that of low pH.
Copyright © 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Structural characterization of alpha-synuclein in an aggregation prone state.Protein Sci. 2009 Sep;18(9):1840-6. doi: 10.1002/pro.194. Protein Sci. 2009. PMID: 19554627 Free PMC article.
-
Charge neutralization and collapse of the C-terminal tail of alpha-synuclein at low pH.Protein Sci. 2009 Jul;18(7):1531-40. doi: 10.1002/pro.149. Protein Sci. 2009. PMID: 19475665 Free PMC article.
-
Alpha-synuclein structures from fluorescence energy-transfer kinetics: implications for the role of the protein in Parkinson's disease.Proc Natl Acad Sci U S A. 2004 Nov 23;101(47):16466-71. doi: 10.1073/pnas.0407307101. Epub 2004 Nov 9. Proc Natl Acad Sci U S A. 2004. PMID: 15536128 Free PMC article.
-
Effects of Mutations and Post-Translational Modifications on α-Synuclein In Vitro Aggregation.J Mol Biol. 2022 Dec 15;434(23):167859. doi: 10.1016/j.jmb.2022.167859. Epub 2022 Oct 19. J Mol Biol. 2022. PMID: 36270580 Free PMC article. Review.
-
Disruptive membrane interactions of alpha-synuclein aggregates.Biochim Biophys Acta Proteins Proteom. 2019 May;1867(5):468-482. doi: 10.1016/j.bbapap.2018.10.006. Epub 2018 Oct 11. Biochim Biophys Acta Proteins Proteom. 2019. PMID: 30315896 Review.
Cited by
-
Function and dysfunction of α-synuclein: probing conformational changes and aggregation by single molecule fluorescence.Mol Neurobiol. 2013 Apr;47(2):622-31. doi: 10.1007/s12035-012-8338-x. Epub 2012 Sep 16. Mol Neurobiol. 2013. PMID: 22983916 Free PMC article. Review.
-
Labeling proteins with fluorophore/thioamide Förster resonant energy transfer pairs by combining unnatural amino acid mutagenesis and native chemical ligation.J Am Chem Soc. 2013 May 1;135(17):6529-40. doi: 10.1021/ja4005943. Epub 2013 Apr 17. J Am Chem Soc. 2013. PMID: 23594264 Free PMC article.
-
Independent tubulin binding and polymerization by the proline-rich region of Tau is regulated by Tau's N-terminal domain.J Biol Chem. 2019 Dec 13;294(50):19381-19394. doi: 10.1074/jbc.RA119.010172. Epub 2019 Nov 7. J Biol Chem. 2019. PMID: 31699899 Free PMC article.
-
Dynamics and dimension of an amyloidogenic disordered state of human β(2)-microglobulin.Eur Biophys J. 2013 Oct;42(10):767-76. doi: 10.1007/s00249-013-0923-z. Epub 2013 Aug 24. Eur Biophys J. 2013. PMID: 23974249
-
A functional role for intrinsic disorder in the tau-tubulin complex.Proc Natl Acad Sci U S A. 2016 Dec 13;113(50):14336-14341. doi: 10.1073/pnas.1610137113. Epub 2016 Nov 23. Proc Natl Acad Sci U S A. 2016. PMID: 27911791 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

