Subunit dissociation and metal binding by Escherichia coli apo-manganese superoxide dismutase
- PMID: 21044611
- PMCID: PMC3018548
- DOI: 10.1016/j.abb.2010.10.021
Subunit dissociation and metal binding by Escherichia coli apo-manganese superoxide dismutase
Abstract
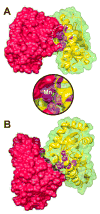
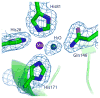
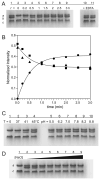
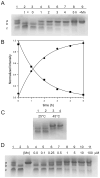
Metal binding by apo-manganese superoxide dismutase (apo-MnSOD) is essential for functional maturation of the enzyme. Previous studies have demonstrated that metal binding by apo-MnSOD is conformationally gated, requiring protein reorganization for the metal to bind. We have now solved the X-ray crystal structure of apo-MnSOD at 1.9Å resolution. The organization of active site residues is independent of the presence of the metal cofactor, demonstrating that protein itself templates the unusual metal coordination geometry. Electrophoretic analysis of mixtures of apo- and (Mn₂)-MnSOD, dye-conjugated protein, or C-terminal Strep-tag II fusion protein reveals a dynamic subunit exchange process associated with cooperative metal binding by the two subunits of the dimeric protein. In contrast, (S126C) (SS) apo-MnSOD, which contains an inter-subunit covalent disulfide-crosslink, exhibits anti-cooperative metal binding. The protein concentration dependence of metal uptake kinetics implies that protein dissociation is involved in metal binding by the wild type apo-protein, although other processes may also contribute to gating metal uptake. Protein concentration dependent small-zone size exclusion chromatography is consistent with apo-MnSOD dimer dissociation at low protein concentration (K(D)=1×10⁻⁵ M). Studies on metal uptake by apo-MnSOD in Escherichia coli cells show that the protein exhibits similar behavior in vivo and in vitro.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
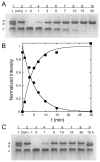


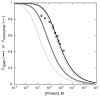
 ) theoretical curve for Fopen, KD = (6.6±0.9)×10−6 M. (
) theoretical curve for Fopen, KD = (6.6±0.9)×10−6 M. ( ) theoretical curve for KD(SEC) = 1.1×10−6 M. (
) theoretical curve for KD(SEC) = 1.1×10−6 M. ( )) bounding limits for 5-fold variation of KD(SEC).
)) bounding limits for 5-fold variation of KD(SEC).

Similar articles
-
In vitro metal uptake by recombinant human manganese superoxide dismutase.Arch Biochem Biophys. 2009 Nov;491(1-2):69-74. doi: 10.1016/j.abb.2009.09.003. Epub 2009 Sep 13. Arch Biochem Biophys. 2009. PMID: 19755112 Free PMC article.
-
Kinetic analysis of the metal binding mechanism of Escherichia coli manganese superoxide dismutase.Biophys J. 2006 Jan 15;90(2):598-607. doi: 10.1529/biophysj.105.071308. Epub 2005 Oct 28. Biophys J. 2006. PMID: 16258041 Free PMC article.
-
Thermally triggered metal binding by recombinant Thermus thermophilus manganese superoxide dismutase, expressed as the apo-enzyme.J Biol Chem. 1999 Dec 3;274(49):34751-7. doi: 10.1074/jbc.274.49.34751. J Biol Chem. 1999. PMID: 10574944
-
The irony of manganese superoxide dismutase.Biochem Soc Trans. 2003 Dec;31(Pt 6):1318-21. doi: 10.1042/bst0311318. Biochem Soc Trans. 2003. PMID: 14641053 Review.
-
Manganese superoxide dismutase.Met Ions Biol Syst. 2000;37:587-611. Met Ions Biol Syst. 2000. PMID: 10693146 Review. No abstract available.
Cited by
-
Battles with iron: manganese in oxidative stress protection.J Biol Chem. 2012 Apr 20;287(17):13541-8. doi: 10.1074/jbc.R111.312181. Epub 2012 Jan 13. J Biol Chem. 2012. PMID: 22247543 Free PMC article. Review.
-
Identifying Metal Binding Sites in Proteins Using Homologous Structures, the MADE Approach.J Chem Inf Model. 2023 Aug 28;63(16):5204-5219. doi: 10.1021/acs.jcim.3c00558. Epub 2023 Aug 9. J Chem Inf Model. 2023. PMID: 37557084 Free PMC article.
-
The mismetallation of enzymes during oxidative stress.J Biol Chem. 2014 Oct 10;289(41):28121-8. doi: 10.1074/jbc.R114.588814. Epub 2014 Aug 26. J Biol Chem. 2014. PMID: 25160623 Free PMC article. Review.
-
Iron incorporation into MnSOD A (bacterial Mn-dependent superoxide dismutase) leads to the formation of a peroxidase/catalase implicated in oxidative damage to bacteria.Biochim Biophys Acta. 2015 Sep;1850(9):1795-805. doi: 10.1016/j.bbagen.2015.05.006. Epub 2015 May 9. Biochim Biophys Acta. 2015. PMID: 25964067 Free PMC article.
-
Metallation state of human manganese superoxide dismutase expressed in Saccharomyces cerevisiae.Arch Biochem Biophys. 2012 Jul 15;523(2):191-7. doi: 10.1016/j.abb.2012.04.016. Epub 2012 Apr 26. Arch Biochem Biophys. 2012. PMID: 22561997 Free PMC article.
References
-
- McCord JM. Oxygen-derived free radicals. New Horiz. 1993;1:70–76. - PubMed
-
- Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. - PubMed
-
- Tainer JA, Getzoff ED, Beem KM, Richardson JS, Richardson DC. Determination and analysis of the 2 Å-structure of copper, zinc superoxide dismutase. J Mol Biol. 1982;160:181–217. - PubMed
-
- Lah MS, Dixon MM, Pattridge KA, Stallings WC, Fee JA, Ludwig ML. Structure-function in Escherichia coli iron superoxide dismutase: comparisons with the manganese enzyme from Thermus thermophilus. Biochemistry. 1995;34:1646–1660. - PubMed
-
- Edwards RA, Baker HM, Whittaker MM, Whittaker JW, Jameson GB, Baker EN. Crystal structure of Escherichia coli manganese superoxide dismutase at 2.1 Å resolution. J Biol Inorg Chem. 1998;3:161–171.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

