Activated protein C N-linked glycans modulate cytoprotective signaling function on endothelial cells
- PMID: 21044954
- PMCID: PMC3020740
- DOI: 10.1074/jbc.M110.159475
Activated protein C N-linked glycans modulate cytoprotective signaling function on endothelial cells
Abstract
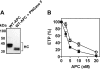
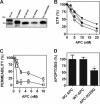
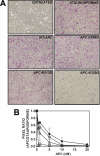
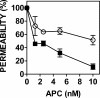
Activated protein C (APC) has potent anticoagulant and anti-inflammatory properties that limit clot formation, inhibit apoptosis, and protect vascular endothelial cell barrier integrity. In this study, the role of N-linked glycans in modulating APC endothelial cytoprotective signaling via endothelial cell protein C receptor/protease-activated receptor 1 (PAR1) was investigated. Enzymatic digestion of APC N-linked glycans (PNG-APC) decreased the APC concentration required to achieve half-maximal inhibition of thrombin-induced endothelial cell barrier permeability by 6-fold. Furthermore, PNG-APC exhibited increased protection against staurosporine-induced endothelial cell apoptosis when compared with untreated APC. To investigate the specific N-linked glycans responsible, recombinant APC variants were generated in which each N-linked glycan attachment site was eliminated. Of these, APC-N329Q was up to 5-fold more efficient in protecting endothelial barrier function when compared with wild type APC. Based on these findings, an APC variant (APC-L38D/N329Q) was generated with minimal anticoagulant activity, but 5-fold enhanced endothelial barrier protective function and 30-fold improved anti-apoptotic function when compared with wild type APC. These data highlight the previously unidentified role of APC N-linked glycosylation in modulating endothelial cell protein C receptor-dependent cytoprotective signaling via PAR1. Furthermore, our data suggest that plasma β-protein C, characterized by aberrant N-linked glycosylation at Asn-329, may be particularly important for maintenance of APC cytoprotective functions in vivo.
Figures


Similar articles
-
A novel protein C-factor VII chimera provides new insights into the structural requirements for cytoprotective protease-activated receptor 1 signaling.J Thromb Haemost. 2017 Nov;15(11):2198-2207. doi: 10.1111/jth.13807. Epub 2017 Sep 21. J Thromb Haemost. 2017. PMID: 28834159
-
Activated protein C promotes protease-activated receptor-1 cytoprotective signaling through β-arrestin and dishevelled-2 scaffolds.Proc Natl Acad Sci U S A. 2011 Dec 13;108(50):E1372-80. doi: 10.1073/pnas.1112482108. Epub 2011 Nov 21. Proc Natl Acad Sci U S A. 2011. PMID: 22106258 Free PMC article.
-
Protease activated receptor 1 (PAR-1) activation by thrombin is protective in human pulmonary artery endothelial cells if endothelial protein C receptor is occupied by its natural ligand.Thromb Haemost. 2008 Jul;100(1):101-9. doi: 10.1160/TH08-02-0127. Thromb Haemost. 2008. PMID: 18612544 Free PMC article.
-
The occupancy of endothelial protein C receptor by its ligand modulates the par-1 dependent signaling specificity of coagulation proteases.IUBMB Life. 2011 Jun;63(6):390-6. doi: 10.1002/iub.447. Epub 2011 Mar 24. IUBMB Life. 2011. PMID: 21438119 Free PMC article. Review.
-
A New Concept of Action of Hemostatic Proteases on Inflammation, Neurotoxicity, and Tissue Regeneration.Biochemistry (Mosc). 2017 Jul;82(7):778-790. doi: 10.1134/S0006297917070033. Biochemistry (Mosc). 2017. PMID: 28918742 Review.
Cited by
-
Cytoprotective-selective activated protein C therapy for ischaemic stroke.Thromb Haemost. 2014 Nov;112(5):883-92. doi: 10.1160/TH14-05-0448. Epub 2014 Sep 18. Thromb Haemost. 2014. PMID: 25230930 Free PMC article. Review.
-
Interleukin-1β-induced autophagy-related gene 5 regulates proliferation of embryonic stem cell-derived odontoblastic cells.PLoS One. 2015 Apr 20;10(4):e0124542. doi: 10.1371/journal.pone.0124542. eCollection 2015. PLoS One. 2015. Retraction in: PLoS One. 2019 Oct 18;14(10):e0224332. doi: 10.1371/journal.pone.0224332. PMID: 25894570 Free PMC article. Retracted.
-
Purification of protein C from canine plasma.BMC Vet Res. 2014 Oct 18;10:251. doi: 10.1186/s12917-014-0251-2. BMC Vet Res. 2014. PMID: 25326145 Free PMC article.
-
Activated Protein C in Cutaneous Wound Healing: From Bench to Bedside.Int J Mol Sci. 2019 Feb 19;20(4):903. doi: 10.3390/ijms20040903. Int J Mol Sci. 2019. PMID: 30791425 Free PMC article. Review.
-
Protein C Pathway in Paediatric and Neonatal Sepsis.Front Pediatr. 2022 Feb 2;9:562495. doi: 10.3389/fped.2021.562495. eCollection 2021. Front Pediatr. 2022. PMID: 35186813 Free PMC article. Review.
References
-
- Owen W. G., Esmon C. T. (1981) J. Biol. Chem. 256, 5532–5535 - PubMed
-
- Fay P. J., Smudzin T. M., Walker F. J. (1991) J. Biol. Chem. 266, 20139–20145 - PubMed
-
- Walker F. J., Sexton P. W., Esmon C. T. (1979) Biochim. Biophys. Acta 571, 333–342 - PubMed
-
- Riewald M., Petrovan R. J., Donner A., Mueller B. M., Ruf W. (2002) Science 296, 1880–1882 - PubMed
-
- White B., Schmidt M., Murphy C., Livingstone W., O'Toole D., Lawler M., O'Neill L., Kelleher D., Schwarz H. P., Smith O. P. (2000) Br. J. Haematol. 110, 130–134 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

