Conserved residues within the putative S4-S5 region serve distinct functions among thermosensitive vanilloid transient receptor potential (TRPV) channels
- PMID: 21044960
- PMCID: PMC3009871
- DOI: 10.1074/jbc.M110.145466
Conserved residues within the putative S4-S5 region serve distinct functions among thermosensitive vanilloid transient receptor potential (TRPV) channels
Abstract
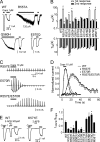
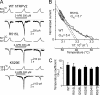
The vanilloid transient receptor potential channel TRPV1 is a tetrameric six-transmembrane segment (S1-S6) channel that can be synergistically activated by various proalgesic agents such as capsaicin, protons, heat, or highly depolarizing voltages, and also by 2-aminoethoxydiphenyl borate (2-APB), a common activator of the related thermally gated vanilloid TRP channels TRPV1, TRPV2, and TRPV3. In these channels, the conserved charged residues in the intracellular S4-S5 region have been proposed to constitute part of a voltage sensor that acts in concert with other stimuli to regulate channel activation. The molecular basis of this gating event is poorly understood. We mutated charged residues all along the S4 and the S4-S5 linker of TRPV1 and identified four potential voltage-sensing residues (Arg(557), Glu(570), Asp(576), and Arg(579)) that, when specifically mutated, altered the functionality of the channel with respect to voltage, capsaicin, heat, 2-APB, and/or their interactions in different ways. The nonfunctional charge-reversing mutations R557E and R579E were partially rescued by the charge-swapping mutations R557E/E570R and D576R/R579E, indicating that electrostatic interactions contribute to allosteric coupling between the voltage-, temperature- and capsaicin-dependent activation mechanisms. The mutant K571E was normal in all aspects of TRPV1 activation except for 2-APB, revealing the specific role of Lys(571) in chemical sensitivity. Surprisingly, substitutions at homologous residues in TRPV2 or TRPV3 had no effect on temperature- and 2-APB-induced activity. Thus, the charged residues in S4 and the S4-S5 linker contribute to voltage sensing in TRPV1 and, despite their highly conserved nature, regulate the temperature and chemical gating in the various TRPV channels in different ways.
Figures
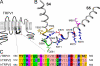
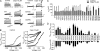
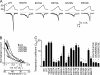
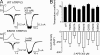
References
-
- Caterina M. J., Schumacher M. A., Tominaga M., Rosen T. A., Levine J. D., Julius D. (1997) Nature 389, 816–824 - PubMed
-
- Voets T., Droogmans G., Wissenbach U., Janssens A., Flockerzi V., Nilius B. (2004) Nature 430, 748–754 - PubMed
-
- Tominaga M., Caterina M. J., Malmberg A. B., Rosen T. A., Gilbert H., Skinner K., Raumann B. E., Basbaum A. I., Julius D. (1998) Neuron 21, 531–543 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

