Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells
- PMID: 21045126
- PMCID: PMC2993408
- DOI: 10.1073/pnas.1014051107
Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells
Abstract
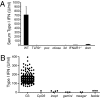
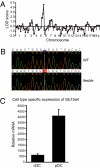
Despite their low frequency, plasmacytoid dendritic cells (pDCs) produce most of the type I IFN that is detectable in the blood following viral infection. The endosomal Toll-like receptors (TLRs) TLR7 and TLR9 are required for pDCs, as well as other cell types, to sense viral nucleic acids, but the mechanism by which signaling through these shared receptors results in the prodigious production of type I IFN by pDCs is not understood. We designed a genetic screen to identify proteins required for the development and specialized function of pDCs. One phenovariant, which we named feeble, showed abrogation of both TLR-induced type I IFN and proinflammatory cytokine production by pDCs, while leaving TLR responses intact in other cells. The feeble phenotype was mapped to a mutation in Slc15a4, which encodes the peptide/histidine transporter 1 (PHT1) and has not previously been implicated in pDC function. The identification of the feeble mutation led to our subsequent observations that AP-3, as well as the BLOC-1 and BLOC-2 Hermansky-Pudlak syndrome proteins are essential for pDC signaling through TLR7 and TLR9. These proteins are not necessary for TLR7 or TLR9 signaling in conventional DCs and thus comprise a membrane trafficking pathway uniquely required for endosomal TLR signaling in pDCs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


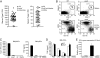
References
-
- Siegal FP, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. - PubMed
-
- Liu YJ. IPC: Professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. - PubMed
-
- Krug A, et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. - PubMed
-
- Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

