Nucleic acid chaperone activity of retroviral Gag proteins
- PMID: 21045546
- PMCID: PMC3073328
- DOI: 10.4161/rna.7.6.13685
Nucleic acid chaperone activity of retroviral Gag proteins
Abstract
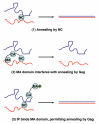
Retrovirus particles in which the Gag protein has not yet been cleaved by the viral protease are termed immature particles. The viral RNA within these particles shows clear evidence of the action of a nucleic acid chaperone (NAC): the genomic RNA is dimeric, and a cellular tRNA molecule is annealed, by its 3' 18 nucleotides, to a complementary stretch in the viral RNA, in preparation for priming reverse transcription in the next round of infection. It seems very likely that the NAC that has catalyzed dimerization and tRNA annealing is the NC domain of the Gag protein itself. However, neither the dimeric linkage nor the tRNA:viral RNA complex has the same structure as those in mature virus particles: thus the conformational effects of Gag within the particles are not equivalent to those of the free NC protein present in mature particles. It is not known whether these dissimilarities reflect intrinsic differences in the NAC activities of Gag and NC, or limitations on Gag imposed by the structure of the immature particle. Analysis of the interactions of recombinant Gag proteins with nucleic acids is complicated by the fact that they result in assembly of virus-like particles. Nevertheless, the available data indicates that the affinity of Gag for nucleic acids can be considerably higher than that of free NC. This enhanced affinity may be due to contributions of the matrix domain, a positively charged region at the N-terminus of Gag; interactions of neighboring Gag molecules with each other may also increase the affinity due to cooperativity of the binding. Recombinant HIV-1 Gag protein clearly exhibits NAC activity. In two well-studied experimental systems, Gag was more efficient than NC, as its NAC effects could be detected at a significantly lower molar ratio of protein to nucleotide than with NC. In one system, binding of nucleic acid by the matrix domain of Gag retarded the Gag-induced annealing of two RNAs; this effect could be ameliorated by the competitive binding of inositol hexakisphosphate to the matrix domain.
Figures
Similar articles
-
Mechanistic differences between nucleic acid chaperone activities of the Gag proteins of Rous sarcoma virus and human immunodeficiency virus type 1 are attributed to the MA domain.J Virol. 2014 Jul;88(14):7852-61. doi: 10.1128/JVI.00736-14. Epub 2014 Apr 30. J Virol. 2014. PMID: 24789780 Free PMC article.
-
The human immunodeficiency virus type 1 Gag polyprotein has nucleic acid chaperone activity: possible role in dimerization of genomic RNA and placement of tRNA on the primer binding site.J Virol. 1999 May;73(5):4251-6. doi: 10.1128/JVI.73.5.4251-4256.1999. J Virol. 1999. PMID: 10196321 Free PMC article.
-
The matrix domain contributes to the nucleic acid chaperone activity of HIV-2 Gag.Retrovirology. 2016 Mar 17;13:18. doi: 10.1186/s12977-016-0245-1. Retrovirology. 2016. PMID: 26987314 Free PMC article.
-
Nucleic-acid-chaperone activity of retroviral nucleocapsid proteins: significance for viral replication.Trends Biochem Sci. 1998 Aug;23(8):297-301. doi: 10.1016/s0968-0004(98)01256-0. Trends Biochem Sci. 1998. PMID: 9757830 Review.
-
Properties and functions of the nucleocapsid protein in virus assembly.RNA Biol. 2010 Nov-Dec;7(6):744-53. doi: 10.4161/rna.7.6.14065. Epub 2010 Nov 1. RNA Biol. 2010. PMID: 21157181 Free PMC article. Review.
Cited by
-
A guanosine-centric mechanism for RNA chaperone function.Science. 2013 Apr 12;340(6129):190-5. doi: 10.1126/science.1230715. Epub 2013 Mar 7. Science. 2013. PMID: 23470731 Free PMC article.
-
HIV-1 RNA genome packaging: it's G-rated.mBio. 2024 Apr 10;15(4):e0086123. doi: 10.1128/mbio.00861-23. Epub 2024 Feb 27. mBio. 2024. PMID: 38411060 Free PMC article. Review.
-
HIV-1 assembly, budding, and maturation.Cold Spring Harb Perspect Med. 2012 Jul;2(7):a006924. doi: 10.1101/cshperspect.a006924. Cold Spring Harb Perspect Med. 2012. PMID: 22762019 Free PMC article. Review.
-
A structure-based mechanism for tRNA and retroviral RNA remodelling during primer annealing.Nature. 2014 Nov 27;515(7528):591-5. doi: 10.1038/nature13709. Epub 2014 Sep 7. Nature. 2014. PMID: 25209668
-
Adaptation of HIV-1/HIV-2 Chimeras with Defects in Genome Packaging and Viral Replication.mBio. 2022 Oct 26;13(5):e0222022. doi: 10.1128/mbio.02220-22. Epub 2022 Aug 29. mBio. 2022. PMID: 36036631 Free PMC article.
References
-
- Vogt VM. Retroviral Virions and Genomes. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Plainview NY: Cold Spring Harbor Laboratory Press; 1997. pp. 27–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
