Features, processing states, and heterologous protein interactions in the modulation of the retroviral nucleocapsid protein function
- PMID: 21045549
- PMCID: PMC3073331
- DOI: 10.4161/rna.7.6.13777
Features, processing states, and heterologous protein interactions in the modulation of the retroviral nucleocapsid protein function
Abstract
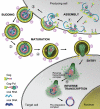
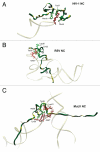
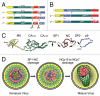
Retroviral nucleocapsid (NC) is central to viral replication. Nucleic acid chaperoning is a key function for NC through the action of its conserved basic amino acids and zinc-finger structures. NC manipulates genomic RNA from its packaging in the producer cell to reverse transcription into the infected host cell. This chaperone function, in conjunction with NC's aggregating properties, is up-modulated by successive NC processing events, from the Gag precursor to the fully mature protein, resulting in the condensation of the nucleocapsid within the capsid shell. Reverse transcription also depends on NC processing, whereas this process provokes NC dissociation from double-stranded DNA, leading to a preintegration complex (PIC), competent for host chromosomal integration. In addition NC interacts with cellular proteins, some of which are involved in viral budding, and also with several viral proteins. All of these properties are reviewed here, focusing on HIV-1 as a paradigmatic reference and highlighting the plasticity of the nucleocapsid architecture.
Figures
Similar articles
-
Retroviral nucleocapsid proteins display nonequivalent levels of nucleic acid chaperone activity.J Virol. 2008 Oct;82(20):10129-42. doi: 10.1128/JVI.01169-08. Epub 2008 Aug 6. J Virol. 2008. PMID: 18684831 Free PMC article.
-
Aromatic residue mutations reveal direct correlation between HIV-1 nucleocapsid protein's nucleic acid chaperone activity and retroviral replication.Virus Res. 2013 Feb;171(2):263-77. doi: 10.1016/j.virusres.2012.07.008. Epub 2012 Jul 16. Virus Res. 2013. PMID: 22814429 Free PMC article.
-
Properties and functions of the nucleocapsid protein in virus assembly.RNA Biol. 2010 Nov-Dec;7(6):744-53. doi: 10.4161/rna.7.6.14065. Epub 2010 Nov 1. RNA Biol. 2010. PMID: 21157181 Free PMC article. Review.
-
The GAG-like protein of the yeast Ty1 retrotransposon contains a nucleic acid chaperone domain analogous to retroviral nucleocapsid proteins.J Biol Chem. 2000 Jun 23;275(25):19210-7. doi: 10.1074/jbc.M001371200. J Biol Chem. 2000. PMID: 10766747
-
Nucleic acid chaperone activity of retroviral Gag proteins.RNA Biol. 2010 Nov-Dec;7(6):700-5. doi: 10.4161/rna.7.6.13685. Epub 2010 Nov 1. RNA Biol. 2010. PMID: 21045546 Free PMC article. Review.
Cited by
-
Determinants of Genomic RNA Encapsidation in the Saccharomyces cerevisiae Long Terminal Repeat Retrotransposons Ty1 and Ty3.Viruses. 2016 Jul 14;8(7):193. doi: 10.3390/v8070193. Viruses. 2016. PMID: 27428991 Free PMC article. Review.
-
The matrix domain contributes to the nucleic acid chaperone activity of HIV-2 Gag.Retrovirology. 2016 Mar 17;13:18. doi: 10.1186/s12977-016-0245-1. Retrovirology. 2016. PMID: 26987314 Free PMC article.
-
APOBEC3G cytosine deamination hotspots are defined by both sequence context and single-stranded DNA secondary structure.Nucleic Acids Res. 2013 Jul;41(12):6139-48. doi: 10.1093/nar/gkt246. Epub 2013 Apr 24. Nucleic Acids Res. 2013. PMID: 23620282 Free PMC article.
-
Comparative nucleic acid chaperone properties of the nucleocapsid protein NCp7 and Tat protein of HIV-1.Virus Res. 2012 Nov;169(2):349-60. doi: 10.1016/j.virusres.2012.06.021. Epub 2012 Jun 26. Virus Res. 2012. PMID: 22743066 Free PMC article. Review.
-
Dynamic interactions of the HIV-1 Tat with nucleic acids are critical for Tat activity in reverse transcription.Nucleic Acids Res. 2014 Jan;42(2):1065-78. doi: 10.1093/nar/gkt934. Epub 2013 Oct 22. Nucleic Acids Res. 2014. PMID: 24153111 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
