Surface characteristics of nanoparticles determine their intracellular fate in and processing by human blood-brain barrier endothelial cells in vitro
- PMID: 21045812
- PMCID: PMC3034845
- DOI: 10.1038/mt.2010.236
Surface characteristics of nanoparticles determine their intracellular fate in and processing by human blood-brain barrier endothelial cells in vitro
Abstract
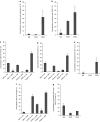
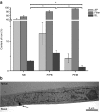
A polarized layer of endothelial cells that comprises the blood-brain barrier (BBB) precludes access of systemically administered medicines to brain tissue. Consequently, there is a need for drug delivery vehicles that mediate transendothelial transport of such medicines. Endothelial cells use a variety of endocytotic pathways for the internalization of exogenous materials, including clathrin-mediated endocytosis, caveolar endocytosis, and macropinocytosis. The different modes of endocytosis result in the delivery of endocytosed material to distinctive intracellular compartments and therewith correlated differential processing. To obtain insight into the properties of drug delivery vehicles that direct their intracellular processing in brain endothelial cells, we investigated the intracellular processing of fixed-size nanoparticles in an in vitro BBB model as a function of distinct nanoparticle surface modifications. Caveolar endocytosis, adsorptive-mediated endocytosis, and receptor-mediated endocytosis were promoted by the use of uncoated 500-nm particles, attachment of the cationic polymer polyethyleneimine (PEI), and attachment of prion proteins, respectively. We demonstrate that surface modifications of nanoparticles, including charge and protein ligands, affect their mode of internalization by brain endothelial cells and thereby their subcellular fate and transcytotic potential.
Figures

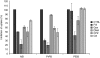

References
-
- Tuma PL., and, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev. 2003;83:871–932. - PubMed
-
- Muro S, Koval M., and, Muzykantov V. Endothelial endocytic pathways: gates for vascular drug delivery. Curr Vasc Pharmacol. 2004;2:281–299. - PubMed
-
- Norkin LC. Caveolae in the uptake and targeting of infectious agents and secreted toxins. Adv Drug Deliv Rev. 2001;49:301–315. - PubMed
-
- Predescu SA, Predescu DN., and, Malik AB. Molecular determinants of endothelial transcytosis and their role in endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2007;293:L823–L842. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

