A phase I dose-escalation study to evaluate safety and tolerability of sorafenib combined with sirolimus in patients with advanced solid cancer
- PMID: 21045832
- PMCID: PMC2994217
- DOI: 10.1038/sj.bjc.6605777
A phase I dose-escalation study to evaluate safety and tolerability of sorafenib combined with sirolimus in patients with advanced solid cancer
Abstract
Background: The combination of sorafenib (vascular endothelial growth factor receptor 2 inhibitor) and sirolimus (mammalian target of rapamycin inhibitor) might work synergistically.
Methods: A phase I dose-escalation study with sorafenib twice a day (b.i.d.) and sirolimus once daily (q.d.) was performed to determine the recommended dose of the combination in patients with solid tumours. Secondary objectives were to determine the safety profile and maximum tolerated dose (MTD), and to evaluate the pharmacokinetics (PK) of the combination.
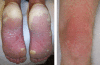
Results: Dose-limiting toxicities were transaminitis and cutaneous toxicity. The most frequently reported adverse events were elevated transaminases, hypophosphatemia, fatigue, anorexia, diarrhoea, nausea, rash and palmar-plantar erythrodysaesthesia. Sirolimus did not change the PK of sorafenib; in contrast, sorafenib reduced the AUC(0-96) and C(max) of sirolimus. No objective responses were observed; eight patients showed stable disease for a median of 16.3 weeks (range 8-24). The MTD of the combination was sorafenib 200 mg b.i.d. with sirolimus 1 mg q.d.
Conclusion: The combination of sorafenib and sirolimus showed enhanced toxicity, which could not be explained by the PK of both drugs. The relative low doses at the MTD, in combination with the PK results, do not warrant further development of this combination.
Figures

References
-
- Abraham RT, Gibbons JJ (2007) The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin Cancer Res 13: 3109–3114 - PubMed
-
- Azad NS, Posadas EM, Kwitkowski VE, Steinberg SM, Jain L, Annunziata CM, Minasian L, Sarosy G, Kotz HL, Premkumar A, Cao L, McNally D, Chow C, Chen HX, Wright JJ, Figg WD, Kohn EC (2008) Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol 26: 3709–3714 - PMC - PubMed
-
- Bhojani N, Jeldres C, Patard JJ, Perrotte P, Suardi N, Hutterer G, Patenaude F, Oudard S, Karakiewicz PI (2008) Toxicities associated with the administration of sorafenib, sunitinib, and temsirolimus and their management in patients with metastatic renal cell carcinoma. Eur Urol 53: 917–930 - PubMed
-
- Cen P, Daleiden A, Doshi G, Amato R (2009) A phase I study of everolimus plus sorafenib in patients with renal cell carcinoma (mRCC). J Clin Oncol 27(e16056): Ref Type: Abstract
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

