Non-thermal plasma induces apoptosis in melanoma cells via production of intracellular reactive oxygen species
- PMID: 21046465
- PMCID: PMC3268344
- DOI: 10.1007/s10439-010-0197-x
Non-thermal plasma induces apoptosis in melanoma cells via production of intracellular reactive oxygen species
Retraction in
-
Retraction note to: Non-thermal plasma induces apoptosis in melanoma cells via production of intracellular reactive oxygen species.Ann Biomed Eng. 2013 Mar;41(3):656. doi: 10.1007/s10439-013-0740-7. Ann Biomed Eng. 2013. PMID: 23314688 Free PMC article. No abstract available.
Abstract
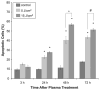
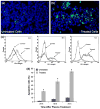
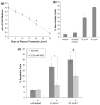
Non-thermal atmospheric pressure dielectric barrier discharge (DBD) plasma may provide a novel approach to treat malignancies via induction of apoptosis. The purpose of this study was to evaluate the potential of DBD plasma to induce apoptosis in melanoma cells. Melanoma cells were exposed to plasma at doses that did not induce necrosis, and cell viability and apoptotic activity were evaluated by Trypan blue exclusion test, Annexin-V/PI staining, caspase-3 cleavage, and TUNEL® analysis. Trypan blue staining revealed that non-thermal plasma treatment significantly decreased the viability of cells in a dose-dependent manner 3 and 24 h after plasma treatment. Annexin-V/PI staining revealed a significant increase in apoptosis in plasma-treated cells at 24, 48, and 72 h post-treatment (p < 0.001). Caspase-3 cleavage was observed 48 h post-plasma treatment at a dose of 15 J/cm(2). TUNEL® analysis of plasma-treated cells demonstrated an increase in apoptosis at 48 and 72 h post-treatment (p < 0.001) at a dose of 15 J/cm(2). Pre-treatment with N-acetyl-L: -cysteine (NAC), an intracellular reactive oxygen species (ROS) scavenger, significantly decreased apoptosis in plasma-treated cells at 5 and 15 J/cm(2). Plasma treatment induces apoptosis in melanoma cells through a pathway that appears to be dependent on production of intracellular ROS. DBD plasma production of intracellular ROS leads to dose-dependent DNA damage in melanoma cells, detected by γ-H2AX, which was completely abrogated by pre-treating cells with ROS scavenger, NAC. Plasma-induced DNA damage in turn may lead to the observed plasma-induced apoptosis. Since plasma is non-thermal, it may be used to selectively treat malignancies.
Figures





References
-
- Berk LB. Radiation therapy as primary and adjuvant treatment for local and regional melanoma. Cancer Control. 2008;15(3):233–238. - PubMed
-
- Colt HG, Crawford SW. In vitro study of the safety limits of bronchoscopic argon plasma coagulation in the presence of airway stents. Respirology. 2006;11(5):643–647. - PubMed
-
- Coulombe S. Live cell permeabilization using the APGD-t. 1st International Conference on Plasma Medicine (ICPM); TX: Corpus Christi; 2007.
-
- Coulombe S, et al. Miniature atmospheric pressure glow discharge torch (APGD-t) for local biomedical applications. Pure Appl Chem. 2006;78(6):1137–1146.
-
- Eliasson B, Egli W, Kogelschatz U. Modelling of dielectric barrier discharge chemistry. Pure Appl Chem. 1994;66(6):1275–1286.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

