Functional heterotypic interactions between astrocyte and oligodendrocyte connexins
- PMID: 21046554
- PMCID: PMC3022368
- DOI: 10.1002/glia.21073
Functional heterotypic interactions between astrocyte and oligodendrocyte connexins
Abstract
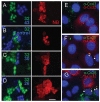
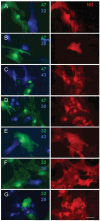
Human genetic diseases and mouse knockouts illustrate that the maintenance of central nervous system myelin requires connexin expression by both astrocytes and oligodendrocytes. Because these cell types express nonoverlapping sets of connexins, the intercellular channels formed between them must be asymmetric with regard to connexin content, defined as heterotypic. Here, we show that oligodendrocyte Cx47 can form heterotypic channels with astrocyte Cx43 or Cx30 but not Cx26, whereas oligodendrocyte Cx32 can functionally interact with astrocyte Cx30 or Cx26 but not Cx43. Thus, as many as four types of intercellular channels could be formed between astrocytes and oligodendrocytes.
© 2010 Wiley-Liss, Inc.
Figures




References
-
- Beltramello M, Bicego M, Piazza V, Ciubotaru CD, Mammano F, D'Andrea P. Permeability and gating properties of human connexins 26 and 30 expressed in HeLa cells. Biochem Biophys Res Commun. 2003;305:1024–1033. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

