Water in the polar and nonpolar cavities of the protein interleukin-1β
- PMID: 21047091
- PMCID: PMC3005849
- DOI: 10.1021/jp108731r
Water in the polar and nonpolar cavities of the protein interleukin-1β
Abstract
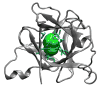
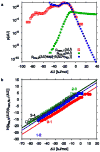
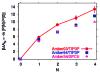
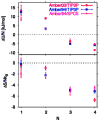
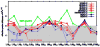
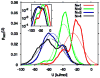
Water in the protein interior serves important structural and functional roles and is also increasingly recognized as a relevant factor in drug binding. The nonpolar cavity in the protein interleukin-1β has been reported to be filled by water on the basis of some experiments and simulations and to be empty on the basis of others. Here we study the thermodynamics of filling the central nonpolar cavity and the four polar cavities of interleukin-1β by molecular dynamics simulation. We use different water models (TIP3P and SPC/E) and protein force fields (amber94 and amber03) to calculate the semigrand partition functions term by term that quantify the hydration equilibria. We consistently find that water in the central nonpolar cavity is thermodynamically unstable, independent of force field and water model. The apparent reason is the relatively small size of the cavity, with a volume less than ∼80 Å(3). Our results are consistent with the most recent X-ray crystallographic and simulation studies but disagree with an earlier interpretation of nuclear magnetic resonance (NMR) experiments probing protein-water interactions. We show that, at least semiquantitatively, the measured nuclear Overhauser effects indicating the proximity of water to the methyl groups lining the nonpolar cavity can, in all likelihood, be attributed to interactions with buried and surface water molecules near the cavity. The same methods applied to determine the occupancy of the polar cavities show that they are filled by the same number of water molecules observed in crystallography, thereby validating the theoretical and simulation methods used to study the water occupancy in the nonpolar protein cavity.
Figures

References
-
- Eriksson AE, Baase WA, Zhang XJ, Heinz DW, Blaber M, Baldwin EP, Matthews BW. Science. 1992;255:178–183. - PubMed
-
- Otting G, Wüthrich K. J Am Chem Soc. 1989;111:1871–1875.
-
- Clore GM, Bax A, Wingfield PT, Gronenborn AM. Biochemistry. 1990;29:5671–5676. - PubMed
-
- Denisov VP, Halle B. Faraday Discuss. 1996;103:227–244. - PubMed
-
- Otting G, Liepinsh E, Halle B, Frey U. Nature Struct Biol. 1997;4:396–404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

