The art of engineering viral nanoparticles
- PMID: 21047140
- PMCID: PMC3156490
- DOI: 10.1021/mp100225y
The art of engineering viral nanoparticles
Abstract
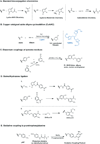
Viral nanotechnology is an emerging and highly interdisciplinary field in which viral nanoparticles (VNPs) are applied in diverse areas such as electronics, energy and next-generation medical devices. VNPs have been developed as candidates for novel materials, and are often described as "programmable" because they can be modified and functionalized using a number of techniques. In this review, we discuss the concepts and methods that allow VNPs to be engineered, including (i) bioconjugation chemistries, (ii) encapsulation techniques, (iii) mineralization strategies, and (iv) film and hydrogel development. With all these techniques in hand, the potential applications of VNPs are limited only by the imagination.
Figures






References
-
- Zaitlin M. The discovery of the causal agent of the tobacco mosaic disease. In: Kung S-D, Yang S-F, editors. Discoveries in Plant Biology. Hong Kong: World Publishing; 1998. pp. 105–110.
-
- Clark JR, March JB. Bacteriophages and biotechnology: vaccines, gene therapy and antibacterials. Trends Biotechnol. 2006;24(5):212–218. - PubMed
-
- Marks T, Sharp R. Bacteriophages and biotechnology: a review. J. Chem. Technol. Biotechnol. 2000;75:6–17.
-
- Garcea RL, Gissmann L. Virus-like particles as vaccines and vessels for the delivery of small molecules. Curr. Opin. Biotechnol. 2004;15(6):513–517. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

