The endogenous soluble VEGF receptor-2 isoform suppresses lymph node metastasis in a mouse immunocompetent mammary cancer model
- PMID: 21047425
- PMCID: PMC2989928
- DOI: 10.1186/1741-7015-8-69
The endogenous soluble VEGF receptor-2 isoform suppresses lymph node metastasis in a mouse immunocompetent mammary cancer model
Abstract
Background: Cancer metastasis contributes significantly to cancer mortality and is facilitated by lymphangiogenesis and angiogenesis. A new splicing variant, endogenous soluble vascular endothelial growth factor receptor-2 (esVEGFR-2) that we recently identified is an endogenous selective inhibitor of lymphangiogenesis. To evaluate the antimetastatic potential of esVEGFR-2, gene therapy with vector expressing esVEGFR-2 (pesVEGFR-2) or endostatin (pEndo) as a positive control was conducted on murine metastatic mammary cancer.
Methods: Syngeneic inoculated metastatic mammary cancers received direct intratumoral injection of pesVEGFR-2, pEndo or pVec as control, once a week for six weeks. In vivo gene electrotransfer was performed on the tumors after each injection.
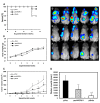
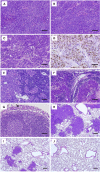
Results: Deaths from metastasis were much lower in the pesVEGFR-2 and pEndo groups than in those of the pVec. Tumor volume was significantly lower in the pesVEGFR-2 and the pEndo groups throughout the study. Multiplicity of lymph node and lung metastatic nodules was significantly suppressed in the pesVEGFR-2 and pEndo groups. Moreover, the total number of overall metastasis including the other organs was also decreased in these groups. However, pesVEGFR-2 was not able to decrease the number of lungs, ovaries, kidneys and adrenals with metastasis as counted by unilateral or bilateral metastasis. The number of CD34+/Lyve-1⁻ blood microvessels was significantly decreased in the pEndo group, while the number of CD34⁻/Lyve-1+ lymphatic vessels was significantly decreased in the pesVEGFR-2 and pEndo groups. In addition, a significant reduction in the number of dilated lymphatic vessels containing intraluminal cancer cells was observed in the pesVEGFR-2 and pEndo groups. Levels of apoptosis were significantly increased in the pEndo group, whereas the rates of cell proliferation were significantly decreased in the pesVEGFR-2 and pEndo groups.
Conclusions: Our data demonstrate that esVEGFR-2 can inhibit mainly lymph node metastasis. The antimetastatic activity of esVEGFR-2 may be of high clinical significance in the treatment of metastatic breast cancer because lymph node involvement is a most important prognostic factor in cancer patients.
Figures



Similar articles
-
Mammary cancer gene therapy targeting lymphangiogenesis: VEGF-C siRNA and soluble VEGF receptor-2, a splicing variant.Med Mol Morphol. 2012 Dec;45(4):179-84. doi: 10.1007/s00795-012-0576-5. Epub 2012 Dec 7. Med Mol Morphol. 2012. PMID: 23224595 Review.
-
Combination therapy with short interfering RNA vectors against VEGF-C and VEGF-A suppresses lymph node and lung metastasis in a mouse immunocompetent mammary cancer model.Cancer Gene Ther. 2008 Dec;15(12):776-86. doi: 10.1038/cgt.2008.43. Epub 2008 Jul 25. Cancer Gene Ther. 2008. PMID: 18654613
-
Electrogene therapy using endostatin, with or without suicide gene therapy, suppresses murine mammary tumor growth and metastasis.Cancer Gene Ther. 2007 Mar;14(3):268-78. doi: 10.1038/sj.cgt.7701009. Epub 2006 Nov 10. Cancer Gene Ther. 2007. PMID: 17096028
-
Therapy with siRNA for Vegf-c but not for Vegf-d suppresses wide-spectrum organ metastasis in an immunocompetent xenograft model of metastatic mammary cancer.Anticancer Res. 2013 Oct;33(10):4237-47. Anticancer Res. 2013. PMID: 24122987
-
Molecular control of lymphatic metastasis.Ann N Y Acad Sci. 2008;1131:225-34. doi: 10.1196/annals.1413.020. Ann N Y Acad Sci. 2008. PMID: 18519975 Review.
Cited by
-
Soluble Vegfr3 gene therapy suppresses multi-organ metastasis in a mouse mammary cancer model.Cancer Sci. 2020 Aug;111(8):2837-2849. doi: 10.1111/cas.14531. Epub 2020 Jul 4. Cancer Sci. 2020. PMID: 32539229 Free PMC article.
-
Adult diffuse gliomas produce mRNA transcripts encoding EGFR isoforms lacking a tyrosine kinase domain.Int J Oncol. 2012 Apr;40(4):1142-52. doi: 10.3892/ijo.2011.1287. Epub 2011 Dec 8. Int J Oncol. 2012. PMID: 22159595 Free PMC article.
-
Altered expression of endogenous soluble vascular endothelial growth factor receptor-2 is involved in the progression of esophageal squamous cell carcinoma.J Histochem Cytochem. 2013 May;61(5):340-7. doi: 10.1369/0022155413480181. Epub 2013 Feb 7. J Histochem Cytochem. 2013. PMID: 23392734 Free PMC article.
-
α-Mangostin extracted from the pericarp of the mangosteen (Garcinia mangostana Linn) reduces tumor growth and lymph node metastasis in an immunocompetent xenograft model of metastatic mammary cancer carrying a p53 mutation.BMC Med. 2011 Jun 3;9:69. doi: 10.1186/1741-7015-9-69. BMC Med. 2011. PMID: 21639868 Free PMC article.
-
Hedgehog signalling pathway orchestrates angiogenesis in triple-negative breast cancers.Br J Cancer. 2017 May 23;116(11):1425-1435. doi: 10.1038/bjc.2017.116. Epub 2017 Apr 25. Br J Cancer. 2017. PMID: 28441382 Free PMC article.
References
-
- Cody HS, Borgen PI, Tan LK. Redefining prognosis in node-negative breast cancer: can sentinel lymph node biopsy raise the threshold for systemic adjuvant therapy? Ann Surg Oncol. 2004;11:227S–230S. - PubMed
-
- He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94:819–825. - PubMed
-
- Lin J, Lalani AS, Harding TC, Gonzalez M, Wu WW, Luan B, Tu GH, Koprivnikar K, VanRoey MJ, He Y, Alitalo K, Jooss K. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res. 2005;65:6901–6909. doi: 10.1158/0008-5472.CAN-05-0408. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

