Modulation of human embryonic stem cell-derived cardiomyocyte growth: a testbed for studying human cardiac hypertrophy?
- PMID: 21047517
- PMCID: PMC3034871
- DOI: 10.1016/j.yjmcc.2010.10.029
Modulation of human embryonic stem cell-derived cardiomyocyte growth: a testbed for studying human cardiac hypertrophy?
Abstract
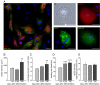
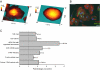
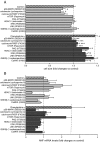
Human embryonic stem cell-derived cardiomyocytes (hESC-CM) are being developed for tissue repair and as a model system for cardiac physiology and pathophysiology. However, the signaling requirements of their growth have not yet been fully characterized. We showed that hESC-CM retain their capacity for increase in size in long-term culture. Exposing hESC-CM to hypertrophic stimuli such as equiaxial cyclic stretch, angiotensin II, and phenylephrine (PE) increased cell size and volume, percentage of hESC-CM with organized sarcomeres, levels of ANF, and cytoskeletal assembly. PE effects on cell size were separable from those on cell cycle. Changes in cell size by PE were completely inhibited by p38-MAPK, calcineurin/FKBP, and mTOR blockers. p38-MAPK and calcineurin were also implicated in basal cell growth. Inhibitors of ERK, JNK, and CaMK II partially reduced PE effects; PKG or GSK3β inhibitors had no effect. The role of p38-MAPK was confirmed by an additional pharmacological inhibitor and adenoviral infection of hESC-CM with a dominant-inhibitory form of p38-MAPK. Infection of hESC-CM with constitutively active upstream MAP2K3b resulted in an increased cell size, sarcomere and cytoskeletal assembly, elongation of the cells, and induction of ANF mRNA levels. siRNA knockdown of p38-MAPK inhibited PE-induced effects on cell size. These results reveal an important role for active protein kinase signaling in hESC-CM growth and hypertrophy, with potential implications for hESC-CM as a novel in vitro test system. This article is part of a special issue entitled, "Cardiovascular Stem Cells Revisited".
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

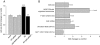

References
-
- Dolnikov K., Shilkrut M., Zeevi-Levin N., Gerecht-Nir S., Amit M., Danon A. Functional properties of human embryonic stem cell-derived cardiomyocytes: intracellular Ca2+ handling and the role of sarcoplasmic reticulum in the contraction. Stem Cells. 2006;24:236–245. - PubMed
-
- Synnergren J., Akesson K., Dahlenborg K., Vidarsson H., Ameen C., Steel D. Molecular signature of cardiomyocyte clusters derived from human embryonic stem cells. Stem Cells. 2008;26:1831–1840. - PubMed
-
- Sartiani L., Bettiol E., Stillitano F., Mugelli A., Cerbai E., Jaconi M.E. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells. 2007;25:1136–1144. - PubMed
-
- Liu J., Fu J.D., Siu C.W., Li R.A. Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell-derived cardiomyocytes: insights for driven maturation. Stem Cells. 2007;25:3038–3044. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/D011027/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- RG/08/007/25296/BHF_/British Heart Foundation/United Kingdom
- G1000035/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- G0600373/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

