Phosphodiesterase 4B is essential for T(H)2-cell function and development of airway hyperresponsiveness in allergic asthma
- PMID: 21047676
- PMCID: PMC3002752
- DOI: 10.1016/j.jaci.2010.08.014
Phosphodiesterase 4B is essential for T(H)2-cell function and development of airway hyperresponsiveness in allergic asthma
Abstract
Background: Cyclic AMP (cAMP) signaling modulates functions of inflammatory cells involved in the pathogenesis of asthma, and type 4 cAMP-specific phosphodiesterases (PDE4s) are essential components of this pathway. Induction of the PDE4 isoform PDE4B is necessary for Toll-like receptor signaling in monocytes and macrophages and is associated with T cell receptor/CD3 in T cells; however, its exact physiological function in the development of allergic asthma remains undefined.
Objectives: We investigated the role of PDE4B in the development of allergen-induced airway hyperresponsiveness (AHR) and T(H)2-driven inflammatory responses.
Methods: Wild-type and PDE4B(-/-) mice were sensitized and challenged with ovalbumin and AHR measured in response to inhaled methacholine. Airway inflammation was characterized by analyzing leukocyte infiltration and cytokine accumulation in the airways. Ovalbumin-stimulated cell proliferation and T(H)2 cytokine production were determined in cultured bronchial lymph node cells.
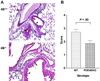
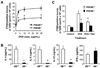
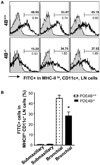
Results: Mice deficient in PDE4B do not develop AHR. This protective effect was associated with a significant decrease in eosinophils recruitment to the lungs and decreased T(H)2 cytokine levels in the bronchoalveolar lavage fluid. Defects in T-cell replication, T(H)2 cytokine production, and dendritic cell migration were evident in cells from the airway-draining lymph nodes. Conversely, accumulation of the T(H)1 cytokine IFN-γ was not affected in PDE4B(-/-) mice. Ablation of the orthologous PDE4 gene PDE4A has no impact on airway inflammation.
Conclusion: By relieving a cAMP-negative constraint, PDE4B plays an essential role in T(H)2-cell activation and dendritic cell recruitment during airway inflammation. These findings provide proof of concept that PDE4 inhibitors with PDE4B selectivity may have efficacy in asthma treatment.
Copyright © 2010 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest: S.-L. C. Jin has received research support from the National Science Council (Taiwan). D. Umetsu has received research support from the National Institutes of Health. M. Conti is a consultant for Pfizer, has received an honorarium from Nycomed, and has received research support from the NIH and the Sandler Foundation. The rest of the authors have declared that they have no conflict of interest.
Figures



Similar articles
-
Effects of ciclamilast, a new PDE 4 PDE4 inhibitor, on airway hyperresponsiveness, PDE4D expression and airway inflammation in a murine model of asthma.Eur J Pharmacol. 2006 Oct 10;547(1-3):125-35. doi: 10.1016/j.ejphar.2006.07.002. Epub 2006 Jul 13. Eur J Pharmacol. 2006. PMID: 16956605
-
Increased Th2 cytokine secretion, eosinophilic airway inflammation, and airway hyperresponsiveness in neurturin-deficient mice.J Immunol. 2011 Jun 1;186(11):6497-504. doi: 10.4049/jimmunol.1001673. Epub 2011 Apr 20. J Immunol. 2011. PMID: 21508262
-
Reduced airway hyperresponsiveness and tracheal responses during allergic asthma in mice lacking tyrosine kinase inducible T-cell kinase.J Allergy Clin Immunol. 2006 Apr;117(4):780-6. doi: 10.1016/j.jaci.2005.12.1330. Epub 2006 Feb 7. J Allergy Clin Immunol. 2006. PMID: 16630934
-
Critical Involvement of CD44 in T Helper Type 2 Cell-Mediated Eosinophilic Airway Inflammation in a Mouse Model of Acute Asthma.Front Immunol. 2022 Jan 7;12:811600. doi: 10.3389/fimmu.2021.811600. eCollection 2021. Front Immunol. 2022. PMID: 35069598 Free PMC article. Review.
-
T cells in asthma: influences of genetics, environment, and T-cell plasticity.J Allergy Clin Immunol. 2013 May;131(5):1267-74; quiz 1275. doi: 10.1016/j.jaci.2013.02.016. Epub 2013 Mar 28. J Allergy Clin Immunol. 2013. PMID: 23541326 Review.
Cited by
-
Asthma Pharmacogenomics: 2015 Update.Curr Allergy Asthma Rep. 2015 Jul;15(7):42. doi: 10.1007/s11882-015-0544-y. Curr Allergy Asthma Rep. 2015. PMID: 26143396 Review.
-
PDE4 as a target for cognition enhancement.Expert Opin Ther Targets. 2013 Sep;17(9):1011-27. doi: 10.1517/14728222.2013.818656. Epub 2013 Jul 25. Expert Opin Ther Targets. 2013. PMID: 23883342 Free PMC article. Review.
-
PDE4B Is a Homeostatic Regulator of Cyclic AMP in Dendritic Cells.Front Pharmacol. 2022 Mar 21;13:833832. doi: 10.3389/fphar.2022.833832. eCollection 2022. Front Pharmacol. 2022. PMID: 35387344 Free PMC article.
-
New Avenues for Phosphodiesterase Inhibitors in Asthma.J Exp Pharmacol. 2021 Mar 15;13:291-302. doi: 10.2147/JEP.S242961. eCollection 2021. J Exp Pharmacol. 2021. PMID: 33758554 Free PMC article. Review.
-
Assessment of PDE4 Inhibitor-Induced Hypothermia as a Correlate of Nausea in Mice.Biology (Basel). 2021 Dec 20;10(12):1355. doi: 10.3390/biology10121355. Biology (Basel). 2021. PMID: 34943270 Free PMC article.
References
-
- Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. - PubMed
-
- Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. - PubMed
-
- Huang S, Xiao H, Kleine-Tebbe J, Paciotti G, Marsh D, Lichtenstein L, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688–2694. - PubMed
-
- Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

