RGS-GAIP-interacting protein controls breast cancer progression
- PMID: 21047775
- PMCID: PMC3850212
- DOI: 10.1158/1541-7786.MCR-10-0209
RGS-GAIP-interacting protein controls breast cancer progression
Abstract
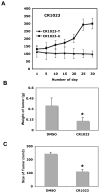
Although the importance of RGS-GAIP-interacting protein (GIPC) in the biology of malignant cells is well known, the molecular mechanism of GIPC in the inhibition of tumor progression has not been identified. This study focused on elucidating the molecular role of GIPC in breast cancer progression. By using a human breast tumor specimen, an in vivo mouse model, and breast cancer cell lines, we showed for the first time that GIPC is involved in breast cancer progression through regulation of breast cancer cell proliferation, survival, and invasion. Furthermore, we found that the Akt/Mdm2/p53 axis, insulin-like growth factor-1 receptor, matrix metalloproteinase-9, and Cdc42 were downstream of GIPC signaling in breast cancer cells. Moreover, we showed that wild-type p53 reduced GIPC-induced breast cancer cell survival, whereas mutant p53 inhibited GIPC-induced cell invasion. Finally, we demonstrated that an N-myristoylated GIPC peptide (CR1023, N-myristoyl-PSQSSSEA) capable of blocking the PDZ domain of GIPC successfully inhibited MDA-MB-231 cell proliferation, survival, and further in vivo tumor growth. Taken together, these findings demonstrate the importance of GIPC in breast tumor progression, which has a potentially significant impact on the development of therapies against many common cancers expressing GIPC, including breast and renal cancer.
©2010 AACR.
Figures












Similar articles
-
Targeting GIPC/synectin in pancreatic cancer inhibits tumor growth.Clin Cancer Res. 2009 Jun 15;15(12):4095-103. doi: 10.1158/1078-0432.CCR-08-2837. Epub 2009 Jun 9. Clin Cancer Res. 2009. PMID: 19509165 Free PMC article.
-
GIPC mediates the generation of reactive oxygen species and the regulation of cancer cell proliferation by insulin-like growth factor-1/IGF-1R signaling.Cancer Lett. 2010 Aug 28;294(2):254-63. doi: 10.1016/j.canlet.2010.02.007. Epub 2010 Mar 4. Cancer Lett. 2010. PMID: 20206441
-
Chemically modified peptides targeting the PDZ domain of GIPC as a therapeutic approach for cancer.ACS Chem Biol. 2012 Apr 20;7(4):770-9. doi: 10.1021/cb200536r. Epub 2012 Feb 15. ACS Chem Biol. 2012. PMID: 22292614 Free PMC article.
-
Strength and duration of GIPC-dependent signaling networks as determinants in cancer.Neoplasia. 2021 Feb;23(2):181-188. doi: 10.1016/j.neo.2020.12.004. Epub 2020 Dec 24. Neoplasia. 2021. PMID: 33360508 Free PMC article. Review.
-
GIPC gene family (Review).Int J Mol Med. 2002 Jun;9(6):585-9. Int J Mol Med. 2002. PMID: 12011974 Review.
Cited by
-
Autocrine functions of VEGF in breast tumor cells: adhesion, survival, migration and invasion.Cell Adh Migr. 2012 Nov-Dec;6(6):547-53. doi: 10.4161/cam.23332. Epub 2012 Nov 1. Cell Adh Migr. 2012. PMID: 23257828 Free PMC article. Review.
-
GAIP interacting protein C-terminus regulates autophagy and exosome biogenesis of pancreatic cancer through metabolic pathways.PLoS One. 2014 Dec 3;9(12):e114409. doi: 10.1371/journal.pone.0114409. eCollection 2014. PLoS One. 2014. PMID: 25469510 Free PMC article.
-
Fatty acid metabolism is related to the immune microenvironment changes of gastric cancer and RGS2 is a new tumor biomarker.Front Immunol. 2022 Dec 14;13:1065927. doi: 10.3389/fimmu.2022.1065927. eCollection 2022. Front Immunol. 2022. PMID: 36591293 Free PMC article.
-
Expansion of GGC Repeat in GIPC1 Is Associated with Oculopharyngodistal Myopathy.Am J Hum Genet. 2020 Jun 4;106(6):793-804. doi: 10.1016/j.ajhg.2020.04.011. Epub 2020 May 14. Am J Hum Genet. 2020. PMID: 32413282 Free PMC article.
-
Synectin promotes fibrogenesis by regulating PDGFR isoforms through distinct mechanisms.JCI Insight. 2017 Dec 21;2(24):e92821. doi: 10.1172/jci.insight.92821. JCI Insight. 2017. PMID: 29263300 Free PMC article.
References
-
- Kirikoshi H, Katoh M. Expression of human GIPC1 in normal tissues, cancer cell lines, and primary tumors. Int J Mol Med. 2002;9:509–13. - PubMed
-
- Muders MH, Dutta SK, Wang L, Lau JS, Bhattacharya R, Smyrk TC, et al. Expression and regulatory role of GAIP-interacting protein GIPC in pancreatic adenocarcinoma. Cancer Research. 2006;66:10264–8. - PubMed
-
- Awan A, Lucic MR, Shaw DM, Sheppard F, Westwater C, Lyons SA, et al. 5T4 interacts with TIP-2/GIPC, a PDZ protein, with implications for metastasis. Biochem Biophys Res Commun. 2002;290:1030–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

