Mesencephalic astrocyte-derived neurotrophic factor (MANF) has a unique mechanism to rescue apoptotic neurons
- PMID: 21047780
- PMCID: PMC3024763
- DOI: 10.1074/jbc.M110.146738
Mesencephalic astrocyte-derived neurotrophic factor (MANF) has a unique mechanism to rescue apoptotic neurons
Abstract
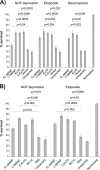
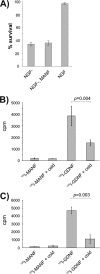
Mesencephalic astrocyte-derived neurotrophic factor (MANF) protects neurons and repairs the Parkinson disease-like symptoms in a rat 6-hydroxydopamine model. We show a three-dimensional solution structure of human MANF that differs drastically from other neurotrophic factors. Remarkably, the C-terminal domain of MANF (C-MANF) is homologous to the SAP domain of Ku70, a well known inhibitor of proapoptotic Bax (Bcl-2-associated X protein). Cellular studies confirm that MANF and C-MANF protect neurons intracellularly as efficiently as Ku70.
Figures


References
-
- Dauer W., Przedborski S. (2003) Neuron 39, 889–909 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

