Inhibition of Arabidopsis O-acetylserine(thiol)lyase A1 by tyrosine nitration
- PMID: 21047785
- PMCID: PMC3013017
- DOI: 10.1074/jbc.M110.147678
Inhibition of Arabidopsis O-acetylserine(thiol)lyase A1 by tyrosine nitration
Abstract
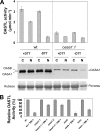
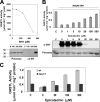
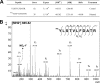
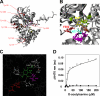
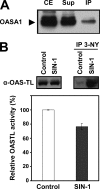
The last step of sulfur assimilation is catalyzed by O-acetylserine(thiol)lyase (OASTL) enzymes. OASTLs are encoded by a multigene family in the model plant Arabidopsis thaliana. Cytosolic OASA1 enzyme is the main source of OASTL activity and thus crucial for cysteine homeostasis. We found that nitrating conditions after exposure to peroxynitrite strongly inhibited OASTL activity. Among OASTLs, OASA1 was markedly sensitive to nitration as demonstrated by the comparative analysis of OASTL activity in nitrated crude protein extracts from wild type and different oastl mutants. Furthermore, nitration assays on purified recombinant OASA1 protein led to 90% reduction of the activity due to inhibition of the enzyme, as no degradation of the protein occurred under these conditions. The reduced activity was due to nitration of the protein because selective scavenging of peroxynitrite with epicatechin impaired OASA1 nitration and the concomitant inhibition of OASTL activity. Inhibition of OASA1 activity upon nitration correlated with the identification of a modified OASA1 protein containing 3-nitroTyr(302) residue. The essential role of the Tyr(302) residue for the catalytic activity was further demonstrated by the loss of OASTL activity of a Y302A-mutated version of OASA1. Inhibition caused by Tyr(302) nitration on OASA1 activity seems to be due to a drastically reduced O-acetylserine substrate binding to the nitrated protein, and also to reduced stabilization of the pyridoxal-5'-phosphate cofactor through hydrogen bonds. This is the first report identifying a Tyr nitration site of a plant protein with functional effect and the first post-translational modification identified in OASA1 enzyme.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

