TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome
- PMID: 21047923
- PMCID: PMC3038481
- DOI: 10.1210/jc.2010-1170
TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome
Abstract
Context and objective: Obesity is associated with activation of the TNF-α system, increased inflammatory markers, and insulin resistance. Although studies in rodents suggest that attenuation of TNF activity improves glucose homeostasis, the effect of prolonged inhibition of TNF-α with etanercept on inflammation and glucose homeostasis in a human model of obesity is not known.
Design and participants: Forty obese subjects with features of metabolic syndrome were randomized to etanercept or placebo, 50 mg twice weekly for 3 months, followed by 50 mg once weekly for 3 months.
Outcome measures: Subjects underwent oral glucose tolerance testing and measurement of serum inflammatory biomarkers and adipokines. Subcutaneous fat biopsy was performed in a subset for measurement of adipokine and TNF-α mRNA expression.
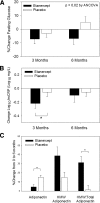
Results: Visceral adiposity was significantly associated with serum concentrations of TNF receptor 1 (TNFR1), TNFR2, and vascular cell adhesion molecule-1 and adipose tissue expression of TNF-α and SOCS-3 (all P < 0.05). Insulin resistance as assessed by homeostasis model assessment was significantly associated with TNFR1, C-reactive protein, IL-6, and soluble intracellular adhesion molecule-1 (sICAM-1) (all P < 0.05). Etanercept significantly improved fasting glucose (treatment effect vs. placebo over 6 months, -10.8 ± 4.4%, P = 0.02). Etanercept also increased the ratio of high molecular weight adiponectin to total adiponectin (+22.1 ± 9.2% vs. placebo, P = 0.02), and decreased levels of sICAM-1 (-11 ± 2% vs. placebo, P < 0.0001). In contrast, body composition, lipids, C-reactive protein, and IL-6 were unchanged after 6 months.
Conclusions: Prolonged therapy with etanercept improved fasting glucose, increased the ratio of high molecular weight to total adiponectin, and decreased sICAM-1 in obese subjects with abnormal glucose homeostasis and significant subclinical inflammation.
Figures
References
-
- Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G 2001 Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 280:E745–E751 - PubMed
-
- Dandona P, Weinstock R, Thusu K, Abdel-Rahman E, Aljada A, Wadden T 1998 Tumor necrosis factor-α in sera of obese patients: fall with weight loss. J Clin Endocrinol Metab 83:2907–2910 - PubMed
-
- Ofei F, Hurel S, Newkirk J, Sopwith M, Taylor R 1996 Effects of an engineered human anti-TNF-α antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes 45:881–885 - PubMed
-
- Paquot N, Castillo MJ, Lefèbvre PJ, Scheen AJ 2000 No increased insulin sensitivity after a single intravenous administration of a recombinant human tumor necrosis factor receptor: Fc fusion protein in obese insulin-resistant patients. J Clin Endocrinol Metab 85:1316–1319 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K23 DK089910/DK/NIDDK NIH HHS/United States
- P30 DK040561/DK/NIDDK NIH HHS/United States
- F32 DK085969/DK/NIDDK NIH HHS/United States
- P01-DK049210/DK/NIDDK NIH HHS/United States
- M01 RR001066/RR/NCRR NIH HHS/United States
- F32 DK085969-01/DK/NIDDK NIH HHS/United States
- P01 DK049210/DK/NIDDK NIH HHS/United States
- K24 DK064545-06/DK/NIDDK NIH HHS/United States
- M01-RR-01066/RR/NCRR NIH HHS/United States
- K23 DK089910-01/DK/NIDDK NIH HHS/United States
- 1 UL1 RR025758-01/RR/NCRR NIH HHS/United States
- F32 DK080642-02/DK/NIDDK NIH HHS/United States
- K24 DK064545/DK/NIDDK NIH HHS/United States
- K23 DK087857/DK/NIDDK NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
- F32 DK080642/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

