The acidic domain of hepatitis C virus NS4A contributes to RNA replication and virus particle assembly
- PMID: 21047963
- PMCID: PMC3020511
- DOI: 10.1128/JVI.01889-10
The acidic domain of hepatitis C virus NS4A contributes to RNA replication and virus particle assembly
Abstract
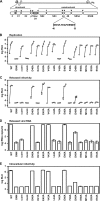
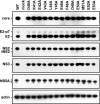
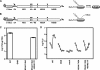
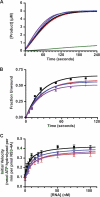
Hepatitis C virus NS3-4A is a membrane-bound enzyme complex that exhibits serine protease, RNA helicase, and RNA-stimulated ATPase activities. This enzyme complex is essential for viral genome replication and has been recently implicated in virus particle assembly. To help clarify the role of NS4A in these processes, we conducted alanine scanning mutagenesis on the C-terminal acidic domain of NS4A in the context of a chimeric genotype 2a reporter virus. Of 13 mutants tested, two (Y45A and F48A) had severe defects in replication, while seven (K41A, L44A, D49A, E50A, M51A, E52A, and E53A) efficiently replicated but had severe defects in virus particle assembly. Multiple strategies were used to identify second-site mutations that suppressed these NS4A defects. The replication defect of NS4A F48A was partially suppressed by mutation of NS4B I7F, indicating that a genetic interaction between NS4A and NS4B contributes to RNA replication. Furthermore, the virus assembly defect of NS4A K41A was suppressed by NS3 Q221L, a mutation previously implicated in overcoming other virus assembly defects. We therefore examined the known enzymatic activities of wild-type or mutant forms of NS3-4A but did not detect specific defects in the mutants. Taken together, our data reveal interactions between NS4A and NS4B that control genome replication and between NS3 and NS4A that control virus assembly.
Figures
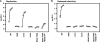

References
-
- Adair, R., et al. 2009. Expression of hepatitis C virus (HCV) structural proteins in trans facilitates encapsidation and transmission of HCV subgenomic RNA. J. Gen. Virol. 90:833-842. - PubMed
-
- Beran, R. K., M. M. Bruno, H. A. Bowers, E. Jankowsky, and A. M. Pyle. 2006. Robust translocation along a molecular monorail: the NS3 helicase from hepatitis C virus traverses unusually large disruptions in its track. J. Mol. Biol. 358:974-982. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

