Total marrow irradiation: a new ablative regimen as part of tandem autologous stem cell transplantation for patients with multiple myeloma
- PMID: 21047977
- PMCID: PMC3717559
- DOI: 10.1158/1078-0432.CCR-10-1912
Total marrow irradiation: a new ablative regimen as part of tandem autologous stem cell transplantation for patients with multiple myeloma
Abstract
Purpose: To establish feasibility, maximum tolerated dose (MTD), and potential efficacy of ablative dose total marrow irradiation (TMI) delivered by helical tomotherapy in patients with multiple myeloma (MM).
Experimental design: Patients with responding or stable MM received tandem autologous stem cell transplants, first with melphalan 200 mg/m(2), and 60 days or later with TMI. TMI doses were to be escalated from 1,000 cGy by increments of 200 cGy. All patients received thalidomide and dexamethasone maintenance.
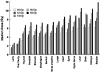
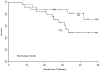
Results: Twenty-two of 25 enrolled patients (79%) received tandem autologous stem cell transplantation (TASCT): TMI was administered at a median of 63.5 days (44-119) after melphalan. Dose-limiting toxicities at level 5 (1,800 cGy) included reversible grade 3 pneumonitis, congestive heart failure, and enteritis (1), and grade 3 hypotension (1). The estimated median radiation dose to normal organs was 11% to 81% of the prescribed marrow dose. Late toxicities included reversible enteritis (1), and lower extremity deep venous thrombosis during maintenance therapy (2). The complete and very good partial response rates were 55% and 27% following TASCT and maintenance therapy. At a median of 35 months of follow-up (21-50+ months), progression-free and overall survival for all patients were 49% (95% CI, 0.27-0.71) and 82% (0.67-1.00).
Conclusion: Ablative dose TMI as part of TASCT is feasible, and the complete response rate is encouraging. Careful monitoring of late toxicities is needed. Further assessment of this modality is justified at the 1,600 cGy MTD level in MM patients who are candidates for ASCT.
©2010 AACR.
Conflict of interest statement
Conflict-of interest disclosure: Jeffrey Wong M.D. is the recipient of a research grant from Tomotherapy Hi-Art System, Madison, WI. The other authors declare no competing conflicts of interests
Figures

References
-
- Lahuerta JJ, Mateos MV, Martinez-Lopez J, et al. Influence of pre- and post-transplantation responses on outcome of patients with multiple myeloma: sequential improvement of response and achievement of complete response are associated with longer survival. J Clin Oncol. 2008;26:5775–82. - PubMed
-
- Harousseau JL, Avet-Loiseau H, Attal M, et al. Achievement of at least very good partial response is a simple and robust prognostic factor in patients with multiple myeloma treated with high-dose therapy: long-term analysis of the IFM 99-02 and 99-04 Trials. J Clin Oncol. 2009;27:5720–6. - PubMed
-
- Chanan-Khan AA, Giralt S. Importance of achieving a complete response in multiple myeloma, and the impact of novel agents. J Clin Oncol. 28:2612–24. - PubMed
-
- Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–83. - PubMed
-
- Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

