Interleukin-6 modulates graft-versus-host responses after experimental allogeneic bone marrow transplantation
- PMID: 21047980
- PMCID: PMC3058832
- DOI: 10.1158/1078-0432.CCR-10-1198
Interleukin-6 modulates graft-versus-host responses after experimental allogeneic bone marrow transplantation
Abstract
Purpose: The graft-versus-tumor (GVT) effect is a potent form of immunotherapy against many hematologic malignancies and some solid tumors. The beneficial GVT effect after allogeneic bone marrow transplantation (BMT) is tightly linked to its most significant complication, graft-versus-host disease (GVHD). The role of interleukin-6 (IL-6) after allogeneic BMT is not well understood. This study used a series of complementary knockout and antibody blockade strategies to analyze the impact of IL-6 in multiple clinically relevant murine models of GVHD and GVT.
Experimental design: We examined the effect of the source of IL-6 by analyzing the role IL-6 deficiency in donor T cells, donor bone marrow or in host tissues. We confirmed and extended the relevance of IL-6 deficiency on GVHD and GVT by treating BMT recipients with anti-mouse IL-6 receptor (IL-6R), MR16-1.
Results: Deficiency of IL-6 in donor T cells led to prolongation of survival. Total inhibition of IL-6 with MR16-1 caused an even greater reduction in GVHD-induced mortality. The reduction in GVHD was independent of the direct effects on T effector cell expansion or donor regulatory T cells. GVT responses were preserved after treatment with MR16-1.
Conclusion: MR16-1 treatment reduced GVHD and preserved sufficient GVT. Tocilizumab, a humanized anti-IL-6R monoclonal antibody (mAb), is approved in several countries including the United States and European Union for the treatment of rheumatoid arthritis and other inflammatory diseases. Blockade of IL-6 with anti-IL-6R mAb therapy may be testable in clinical trials as an adjunct to prevent GVHD in BMT patients without a significant loss of GVT.
©2010 AACR.
Figures

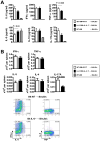
 , n=6), 10×106 wild type (WT) B6 TCDBM and 5×105 WT B6 whole T cells (■, n=12–20) or 10×106 IL-6−/− B6 TCDBM and 5×105 IL-6−/− whole T cells (□, n=12–20). Sera were collected from recipients on day 7. Cytokines were determined by cytokine bead array. (B). Splenocytes from day 7 recipient (□, WT B6 donor, ■ IL-6−/− B6 donor, n=20 respectively) were stimulated with soluble anti-CD3 mAb (2 μg/ml) in the presence of brefeldin A (1/1000 dilution; BioLegend) for 4 hours. Cells were processed for intracellular cytokine staining (ICC), per the manufacturer’s protocol (BD Cytofix/Cytoperm Kit; BD Pharmingen). Representative plots of cytokine expression gated on donor (H-2Kb-positive) CD4 T cells were shown.
, n=6), 10×106 wild type (WT) B6 TCDBM and 5×105 WT B6 whole T cells (■, n=12–20) or 10×106 IL-6−/− B6 TCDBM and 5×105 IL-6−/− whole T cells (□, n=12–20). Sera were collected from recipients on day 7. Cytokines were determined by cytokine bead array. (B). Splenocytes from day 7 recipient (□, WT B6 donor, ■ IL-6−/− B6 donor, n=20 respectively) were stimulated with soluble anti-CD3 mAb (2 μg/ml) in the presence of brefeldin A (1/1000 dilution; BioLegend) for 4 hours. Cells were processed for intracellular cytokine staining (ICC), per the manufacturer’s protocol (BD Cytofix/Cytoperm Kit; BD Pharmingen). Representative plots of cytokine expression gated on donor (H-2Kb-positive) CD4 T cells were shown.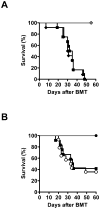


References
-
- Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411:385–9. - PubMed
-
- Wu CJ, Ritz J. Induction of tumor immunity following allogeneic stem cell transplantation. Adv Immunol. 2006;90:133–73. - PubMed
-
- Bleakley M, Riddell SR. Molecules and mechanisms of the graft-versus-leukaemia effect. Nat Rev Cancer. 2004;4:371–80. - PubMed
-
- Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

