Intranasal vaccination with chitosan-DNA nanoparticles expressing pneumococcal surface antigen a protects mice against nasopharyngeal colonization by Streptococcus pneumoniae
- PMID: 21047997
- PMCID: PMC3019786
- DOI: 10.1128/CVI.00263-10
Intranasal vaccination with chitosan-DNA nanoparticles expressing pneumococcal surface antigen a protects mice against nasopharyngeal colonization by Streptococcus pneumoniae
Abstract
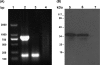
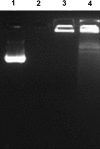
Streptococcus pneumoniae is a respiratory pathogen, and mucosal immune response plays a significant role in the defense against pneumococcal infections. Thus, intranasal vaccination may be an alternative approach to current immunization strategies, and effective delivery systems to mucosal organism are necessary. In this study, BALB/c mice were immunized intranasally with chitosan-DNA nanoparticles expressing pneumococcal surface antigen A (PsaA). Compared to levels in mice immunized with naked DNA or chitosan-pVAX1, anti-PsaA IgG antibody in serum and anti-IgA antibody in mucosal lavages were elevated significantly in mice immunized with chitosan-psaA. The balanced IgG1/IgG2a antibody ratio in serum, enhanced gamma interferon (IFN-γ) and IL-17A levels in spleen lymphocytes, and mucosal washes of mice immunized with chitosan-psaA suggested that cellular immune responses were induced. Furthermore, significantly fewer pneumococci were recovered from the nasopharynx of mice immunized with chitosan-psaA than for the control group following intranasal challenge with ATCC 6303 (serotype 3). These results demonstrated that mucosal immunization with chitosan-psaA may successfully generate mucosal and systemic immune responses and prevent pneumococcal nasopharyngeal colonization. Hence, a chitosan-DNA nanoparticle vaccine expressing pneumococcal major immunodominant antigens after intranasal administration could be developed to prevent pneumococcal infections.
Figures
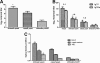
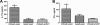


References
-
- Alpar, H. O., S. Somavarapu, K. N. Atuah, and V. W. Bramwell. 2005. Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv. Drug Deliv. Rev. 57:411-430. - PubMed
-
- Arêas, A. P., M. L. Oliveira, E. N. Miyaji, L. C. Leite, K. A. Aires, W. O. Dias, and P. L. Ho. 2004. Expression and characterization of cholera toxin B-pneumococcal surface adhesin A fusion protein in Escherichia coli: ability of CTB-PsaA to induce humoral immune response in mice. Biochem. Biophys. Res. Commun. 321:192-196. - PubMed
-
- Aspden, T. J., J. D. Mason, N. S. Jones, J. Lowe, O. Skaugrud, and L. Illum. 1997. Chitosan as a nasal delivery system: the effect of chitosan solutions on in vitro and in vivo mucociliary transport rates in human turbinates and volunteers. J. Pharm. Sci. 86:509-513. - PubMed
-
- Basset, A., C. M. Thompson, S. K. Hollingshead, D. E. Briles, E. W. Ades, M. Lipsitch, and R. Malley. 2007. Antibody-independent, CD4+ T-cell-dependent protection against pneumococcal colonization elicited by intranasal immunization with purified pneumococcal proteins. Infect. Immun. 75:5460-5464. - PMC - PubMed
-
- Bernatoniene, J., and A. Finn. 2005. Advances in pneumococcal vaccines: advantages for infants and children. Drugs 65:229-255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

