Aryl hydrocarbon receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier
- PMID: 21048045
- PMCID: PMC3023393
- DOI: 10.1096/fj.10-169227
Aryl hydrocarbon receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier
Abstract
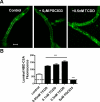
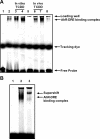
Many widespread and persistent organic pollutants, e.g., 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), activate the aryl hydrocarbon receptor (AhR), causing it to translocate to the cell nucleus, where it transactivates target genes. AhR's ability to target the blood-brain barrier is essentially unexplored. We show here that exposing isolated rat brain capillaries to 0.05-0.5 nM TCDD roughly doubled transport activity and protein expression of P-glycoprotein, an ATP-driven drug efflux pump and a critical determinant of drug entry into the CNS. These effects were abolished by actinomycin D or cycloheximide or by the AhR antagonists resveratrol and α-naphthoflavone. Brain capillaries from TCDD-dosed rats (1-5 μg/kg, i.p.) exhibited increased transport activity and protein expression of 3 xenobiotic efflux pumps, P-glycoprotein, multidrug resistance-associated protein 2, and breast cancer resistance polypeptide, as well as expression of Cyp1a1 and Cyp1b1, both AhR target genes. Consistent with increased P-glycoprotein expression in capillaries from TCDD-dosed rats, in situ brain perfusion indicated significantly reduced brain accumulation of verapamil, a P-glycoprotein substrate. These findings suggest a new paradigm for the field of environmental toxicology: toxicants acting through AhR to target xenobiotic efflux transporters at the blood-brain barrier and thus reduce brain accumulation of CNS-acting therapeutic drugs.
Figures





Similar articles
-
Aryl hydrocarbon receptor ligands increase ABC transporter activity and protein expression in killifish (Fundulus heteroclitus) renal proximal tubules.Biol Chem. 2019 Sep 25;400(10):1335-1345. doi: 10.1515/hsz-2018-0425. Biol Chem. 2019. PMID: 30913027
-
Knockout of the aryl hydrocarbon receptor results in distinct hepatic and renal phenotypes in rats and mice.Toxicol Appl Pharmacol. 2013 Oct 15;272(2):503-18. doi: 10.1016/j.taap.2013.06.024. Epub 2013 Jul 13. Toxicol Appl Pharmacol. 2013. PMID: 23859880
-
A novel 4 S [3H]beta-naphthoflavone-binding protein in liver cytosol of female Sprague-Dawley rats treated with aryl hydrocarbon receptor agonists.Biochem J. 2000 May 1;347 Pt 3(Pt 3):787-95. Biochem J. 2000. PMID: 10769184 Free PMC article.
-
Functional role of AhR in the expression of toxic effects by TCDD.Biochim Biophys Acta. 2003 Feb 17;1619(3):263-8. doi: 10.1016/s0304-4165(02)00485-3. Biochim Biophys Acta. 2003. PMID: 12573486 Review.
-
The aryl hydrocarbon receptor: multitasking in the immune system.Annu Rev Immunol. 2014;32:403-32. doi: 10.1146/annurev-immunol-032713-120245. Annu Rev Immunol. 2014. PMID: 24655296 Review.
Cited by
-
Form and Function of the Vertebrate and Invertebrate Blood-Brain Barriers.Int J Mol Sci. 2021 Nov 9;22(22):12111. doi: 10.3390/ijms222212111. Int J Mol Sci. 2021. PMID: 34829989 Free PMC article. Review.
-
Transcriptomic responses of the clam Meretrix meretrix to the organophosphorus pesticide (dimethoate).Ecotoxicology. 2019 Jul;28(5):539-549. doi: 10.1007/s10646-019-02051-z. Epub 2019 May 22. Ecotoxicology. 2019. PMID: 31119591
-
Novel understanding of ABC transporters ABCB1/MDR/P-glycoprotein, ABCC2/MRP2, and ABCG2/BCRP in colorectal pathophysiology.World J Gastroenterol. 2015 Nov 7;21(41):11862-76. doi: 10.3748/wjg.v21.i41.11862. World J Gastroenterol. 2015. PMID: 26557010 Free PMC article.
-
Obstacles to Brain Tumor Therapy: Key ABC Transporters.Int J Mol Sci. 2017 Nov 27;18(12):2544. doi: 10.3390/ijms18122544. Int J Mol Sci. 2017. PMID: 29186899 Free PMC article. Review.
-
Disruption of small molecule transporter systems by Transporter-Interfering Chemicals (TICs).FEBS Lett. 2020 Dec;594(23):4158-4185. doi: 10.1002/1873-3468.14005. Epub 2020 Dec 9. FEBS Lett. 2020. PMID: 33222203 Free PMC article. Review.
References
-
- Abbott N. J., Patabendige A. A., Dolman D. E., Yusof S. R., Begley D. J. (2009) Structure and function of the blood-brain barrier. Neurobiol. Dis. 37, 13–25 - PubMed
-
- Bauer B., Yang X., Hartz A. M., Olson E. R., Zhao R., Kalvass J. C., Pollack G. M., Miller D. S. (2006) In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol. Pharmacol. 70, 1212–1219 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

