Antibodies protect against intracellular bacteria by Fc receptor-mediated lysosomal targeting
- PMID: 21048081
- PMCID: PMC2996673
- DOI: 10.1073/pnas.1013827107
Antibodies protect against intracellular bacteria by Fc receptor-mediated lysosomal targeting
Abstract
The protective effect of antibodies (Abs) is generally attributed to neutralization or complement activation. Using Legionella pneumophila and Mycobacterium bovis bacillus Calmette-Guérin as a model, we discovered an additional mechanism of Ab-mediated protection effective against intracellular pathogens that normally evade lysosomal fusion. We show that Fc receptor (FcR) engagement by Abs, which can be temporally and spatially separated from bacterial infection, renders the host cell nonpermissive for bacterial replication and targets the pathogens to lysosomes. This process is strictly dependent on kinases involved in FcR signaling but not on host cell protein synthesis or protease activation. Based on these findings, we propose a mechanism whereby Abs and FcR engagement subverts the strategies by which intracellular bacterial pathogens evade lysosomal degradation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


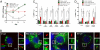
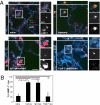
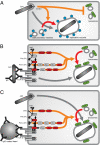
References
-
- Flannagan RS, Cosío G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 2009;7:355–366. - PubMed
-
- McDade JE, et al. Legionnaires’ disease: Isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med. 1977;297:1197–1203. - PubMed
-
- Vogel JP, Andrews HL, Wong SK, Isberg RR. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

