A specific class of interneuron mediates inhibitory plasticity in the lateral amygdala
- PMID: 21048119
- PMCID: PMC6633620
- DOI: 10.1523/JNEUROSCI.3252-10.2010
A specific class of interneuron mediates inhibitory plasticity in the lateral amygdala
Abstract
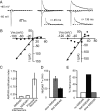
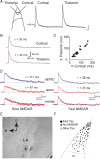
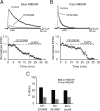
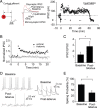
The lateral amygdala (LA) plays a key role in emotional learning and is the main site for sensory input into the amygdala. Within the LA, pyramidal neurons comprise the major cell population with plasticity of inputs to these neurons thought to underlie fear learning. Pyramidal neuron activity is tightly controlled by local interneurons, and GABAergic modulation strongly influences amygdala-dependent learning. Synaptic inputs to some interneurons in the LA can also undergo synaptic plasticity, but the identity of these cells and the mechanisms that underlie this plasticity are not known. Here we show that long-term potentiation (LTP) in LA interneurons is restricted to a specific type of interneuron that is defined by the lack of expression of synaptic NR2B subunits. We find that LTP is only present at cortical inputs to these cells and is initiated by calcium influx via calcium-permeable AMPA receptors. LTP is maintained by trafficking of GluR2-lacking AMPA receptors that require an interaction with SAP97 and the actin cytoskeleton. Our results define a novel population of interneurons in the LA that control principal neuron excitability by feed-forward inhibition of cortical origin. This selective enhanced inhibition may contribute to reducing the activity of principal neurons engaged during extinction of conditioned fear.
Figures





Similar articles
-
Heterosynaptic long-term potentiation of inhibitory interneurons in the lateral amygdala.J Neurosci. 2004 Oct 27;24(43):9507-12. doi: 10.1523/JNEUROSCI.3567-04.2004. J Neurosci. 2004. PMID: 15509737 Free PMC article.
-
Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala.Nature. 1998 Aug 13;394(6694):683-7. doi: 10.1038/29312. Nature. 1998. PMID: 9716132
-
Long-term potentiation at excitatory synaptic inputs to the intercalated cell masses of the amygdala.Int J Neuropsychopharmacol. 2014 Aug;17(8):1233-42. doi: 10.1017/S1461145714000133. Epub 2014 Feb 20. Int J Neuropsychopharmacol. 2014. PMID: 24556032
-
LTP and LTD in cortical GABAergic interneurons: emerging rules and roles.Neuropharmacology. 2011 Apr;60(5):712-9. doi: 10.1016/j.neuropharm.2010.12.020. Epub 2010 Dec 23. Neuropharmacology. 2011. PMID: 21185319 Review.
-
Role of microcircuit structure and input integration in hippocampal interneuron recruitment and plasticity.Neuropharmacology. 2011 Apr;60(5):730-9. doi: 10.1016/j.neuropharm.2010.12.017. Epub 2010 Dec 30. Neuropharmacology. 2011. PMID: 21195097 Review.
Cited by
-
The Basolateral Amygdala: The Core of a Network for Threat Conditioning, Extinction, and Second-Order Threat Conditioning.Biology (Basel). 2023 Sep 22;12(10):1274. doi: 10.3390/biology12101274. Biology (Basel). 2023. PMID: 37886984 Free PMC article. Review.
-
Ex vivo dissection of optogenetically activated mPFC and hippocampal inputs to neurons in the basolateral amygdala: implications for fear and emotional memory.Front Behav Neurosci. 2014 Mar 5;8:64. doi: 10.3389/fnbeh.2014.00064. eCollection 2014. Front Behav Neurosci. 2014. PMID: 24634648 Free PMC article.
-
Specific behaviors during auditory fear conditioning and postsynaptic expression of AMPA receptors in the basolateral amygdala predict interindividual differences in fear generalization in male rats.Learn Mem. 2023 Apr 14;30(4):74-84. doi: 10.1101/lm.053612.122. Print 2023 Apr. Learn Mem. 2023. PMID: 37059464 Free PMC article.
-
Nigrostriatal dopamine modulates the striatal-amygdala pathway in auditory fear conditioning.Nat Commun. 2023 Nov 9;14(1):7231. doi: 10.1038/s41467-023-43066-9. Nat Commun. 2023. PMID: 37945595 Free PMC article.
-
Arc expression identifies the lateral amygdala fear memory trace.Mol Psychiatry. 2016 Mar;21(3):364-75. doi: 10.1038/mp.2015.18. Epub 2015 Mar 24. Mol Psychiatry. 2016. PMID: 25802982 Free PMC article.
References
-
- Bissiere S, Humeau Y, Luthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci. 2003;6:587–592. - PubMed
-
- Chizh BA, Headley PM, Tzschentke TM. NMDA receptor antagonists as analgesics: focus on the NR2B subtype. Trends Pharmacol Sci. 2001;22:636–642. - PubMed
-
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. - PubMed
-
- Davis M. Diazepam and flurazepam: effects on conditioned fear as measured with the potentiated startle paradigm. Psychopharmacology (Berl) 1979;62:1–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
