Supplementary motor area exerts proactive and reactive control of arm movements
- PMID: 21048123
- PMCID: PMC2990193
- DOI: 10.1523/JNEUROSCI.2669-10.2010
Supplementary motor area exerts proactive and reactive control of arm movements
Abstract
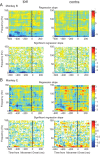
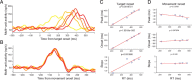
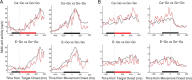
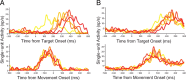
Adaptive behavior requires the ability to flexibly control actions. This can occur either proactively to anticipate task requirements, or reactively in response to sudden changes. Here we report neuronal activity in the supplementary motor area (SMA) that is correlated with both forms of behavioral control. Single-unit and multiunit activity and intracranial local field potentials (LFPs) were recorded in macaque monkeys during a stop-signal task, which elicits both proactive and reactive behavioral control. The LFP power in high- (60-150 Hz) and low- (25-40 Hz) frequency bands was significantly correlated with arm movement reaction time, starting before target onset. Multiunit and single-unit activity also showed a significant regression with reaction time. In addition, LFPs and multiunit and single-unit activity changed their activity level depending on the trial history, mirroring adjustments on the behavioral level. Together, these findings indicate that neuronal activity in the SMA exerts proactive control of arm movements by adjusting the level of motor readiness. On trials when the monkeys successfully canceled arm movements in response to an unforeseen stop signal, the LFP power, particularly in a low (10-50 Hz) frequency range, increased early enough to be causally related to the inhibition of the arm movement on those trials. This indicated that neuronal activity in the SMA is also involved in response inhibition in reaction to sudden task changes. Our findings indicate, therefore, that SMA plays a role in the proactive control of motor readiness and the reactive inhibition of unwanted movements.
Figures

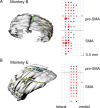
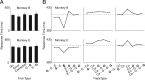

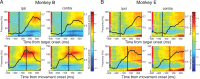

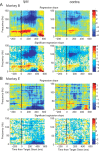





Similar articles
-
Changing directions of forthcoming arm movements: neuronal activity in the presupplementary and supplementary motor area of monkey cerebral cortex.J Neurophysiol. 1996 Oct;76(4):2327-42. doi: 10.1152/jn.1996.76.4.2327. J Neurophysiol. 1996. PMID: 8899607
-
Decoding 3D reach and grasp from hybrid signals in motor and premotor cortices: spikes, multiunit activity, and local field potentials.J Neurophysiol. 2012 Mar;107(5):1337-55. doi: 10.1152/jn.00781.2011. Epub 2011 Dec 7. J Neurophysiol. 2012. PMID: 22157115 Free PMC article.
-
Timing of bimanual movements in human and non-human primates in relation to neuronal activity in primary motor cortex and supplementary motor area.Exp Brain Res. 2002 Oct;146(3):322-35. doi: 10.1007/s00221-002-1174-x. Epub 2002 Aug 17. Exp Brain Res. 2002. PMID: 12232689
-
Role of primate basal ganglia and frontal cortex in the internal generation of movements. III. Neuronal activity in the supplementary motor area.Exp Brain Res. 1992;91(3):396-407. doi: 10.1007/BF00227836. Exp Brain Res. 1992. PMID: 1483514
-
Bereitschaftspotential as an indicator of movement preparation in supplementary motor area and motor cortex.Ciba Found Symp. 1987;132:231-50. doi: 10.1002/9780470513545.ch14. Ciba Found Symp. 1987. PMID: 3322717 Review.
Cited by
-
Supplementary and Premotor Aspects of the Corticospinal Tract Show Links with Restricted and Repetitive Behaviors in Middle-Aged Adults with Autism Spectrum Disorder.Cereb Cortex. 2021 Jul 5;31(8):3962-3972. doi: 10.1093/cercor/bhab062. Cereb Cortex. 2021. PMID: 33791751 Free PMC article.
-
Expectations and violations: delineating the neural network of proactive inhibitory control.Hum Brain Mapp. 2013 Sep;34(9):2015-24. doi: 10.1002/hbm.22047. Epub 2012 Feb 22. Hum Brain Mapp. 2013. PMID: 22359406 Free PMC article.
-
Inhibitory control in mind and brain 2.0: blocked-input models of saccadic countermanding.Psychol Rev. 2015 Apr;122(2):115-47. doi: 10.1037/a0038893. Epub 2015 Feb 23. Psychol Rev. 2015. PMID: 25706403 Free PMC article.
-
Detection of self-paced reaching movement intention from EEG signals.Front Neuroeng. 2012 Jul 12;5:13. doi: 10.3389/fneng.2012.00013. eCollection 2012. Front Neuroeng. 2012. PMID: 23055968 Free PMC article.
-
Intended arm use influences interhemispheric correlation of β-oscillations in primate medial motor areas.J Neurophysiol. 2017 Nov 1;118(5):2865-2883. doi: 10.1152/jn.00379.2016. Epub 2017 Aug 30. J Neurophysiol. 2017. PMID: 28855290 Free PMC article.
References
-
- Benjamini Y, Yekutieli Y. The control of the false discovery rate under dependency. Ann Stat. 2001;29:1165–1188.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
