Reelin signals through apolipoprotein E receptor 2 and Cdc42 to increase growth cone motility and filopodia formation
- PMID: 21048135
- PMCID: PMC3523719
- DOI: 10.1523/JNEUROSCI.4036-10.2010
Reelin signals through apolipoprotein E receptor 2 and Cdc42 to increase growth cone motility and filopodia formation
Abstract
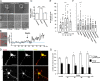
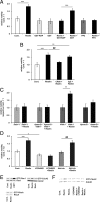
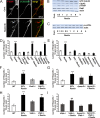
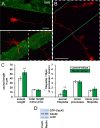
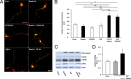
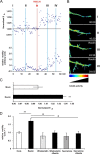
Lipoprotein receptor signaling regulates the positioning and differentiation of postmitotic neurons during development and modulates neuronal plasticity in the mature brain. Depending on the contextual situation, the lipoprotein receptor ligand Reelin can have opposing effects on cortical neurons. We show that Reelin increases growth cone motility and filopodia formation, and identify the underlying signaling cascade. Reelin activates the Rho GTPase Cdc42, known for its role in neuronal morphogenesis and directed migration, in an apolipoprotein E receptor 2-, Disabled-1-, and phosphatidylinositol 3-kinase-dependent manner. We demonstrate that neuronal vesicle trafficking, a Cdc42-controlled process, is increased after Reelin treatment and further provide evidence that the peptidergic VIP/PACAP38 system and Reelin can functionally interact to promote axonal branching. In conclusion, Reelin-induced activation of Cdc42 contributes to the regulation of the cytoskeleton of individual responsive neurons and converges with other signaling cascades to orchestrate Rho GTPase activity and promote neuronal development. Our data link the observation that defects in Rho GTPases and Reelin signaling are responsible for developmental defects leading to neurological and psychiatric disorders.
Figures



Similar articles
-
Reelin induces EphB activation.Cell Res. 2013 Apr;23(4):473-90. doi: 10.1038/cr.2013.7. Epub 2013 Jan 15. Cell Res. 2013. PMID: 23318582 Free PMC article.
-
Reelin stabilizes the actin cytoskeleton of neuronal processes by inducing n-cofilin phosphorylation at serine3.J Neurosci. 2009 Jan 7;29(1):288-99. doi: 10.1523/JNEUROSCI.2934-08.2009. J Neurosci. 2009. PMID: 19129405 Free PMC article.
-
Contribution of the Reelin signaling pathways to nociceptive processing.Eur J Neurosci. 2008 Feb;27(3):523-37. doi: 10.1111/j.1460-9568.2008.06056.x. Eur J Neurosci. 2008. PMID: 18279306
-
Regulation of cortical neuron migration by the Reelin signaling pathway.Neurochem Res. 2011 Jul;36(7):1270-9. doi: 10.1007/s11064-011-0407-4. Epub 2011 Jan 21. Neurochem Res. 2011. PMID: 21253854 Review.
-
[Corticohistogenesis and Reelin signal cascade].Nihon Shinkei Seishin Yakurigaku Zasshi. 2000 Oct;20(4):169-74. Nihon Shinkei Seishin Yakurigaku Zasshi. 2000. PMID: 11215402 Review. Japanese.
Cited by
-
Reelin promotes microtubule dynamics in processes of developing neurons.Histochem Cell Biol. 2013 Feb;139(2):283-97. doi: 10.1007/s00418-012-1025-1. Epub 2012 Sep 19. Histochem Cell Biol. 2013. PMID: 22990595
-
BIG2-ARF1-RhoA-mDia1 Signaling Regulates Dendritic Golgi Polarization in Hippocampal Neurons.Mol Neurobiol. 2018 Oct;55(10):7701-7716. doi: 10.1007/s12035-018-0954-7. Epub 2018 Feb 17. Mol Neurobiol. 2018. PMID: 29455446
-
Optogenetic control of the Dab1 signaling pathway.Sci Rep. 2017 Mar 8;7:43760. doi: 10.1038/srep43760. Sci Rep. 2017. PMID: 28272509 Free PMC article.
-
Nerve growth factor-induced formation of axonal filopodia and collateral branches involves the intra-axonal synthesis of regulators of the actin-nucleating Arp2/3 complex.J Neurosci. 2012 Dec 5;32(49):17671-89. doi: 10.1523/JNEUROSCI.1079-12.2012. J Neurosci. 2012. PMID: 23223289 Free PMC article.
-
Characterization and distribution of Reelin-positive interneuron subtypes in the rat barrel cortex.Cereb Cortex. 2014 Nov;24(11):3046-58. doi: 10.1093/cercor/bht161. Epub 2013 Jun 26. Cereb Cortex. 2014. PMID: 23803971 Free PMC article.
References
-
- Arnaud L, Ballif BA, Förster E, Cooper JA. Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr Biol. 2003a;13:9–17. - PubMed
-
- Assadi AH, Zhang G, Beffert U, McNeil RS, Renfro AL, Niu S, Quattrocchi CC, Antalffy BA, Sheldon M, Armstrong DD, Wynshaw-Boris A, Herz J, D'Arcangelo G, Clark GD. Interaction of reelin signaling and Lis1 in brain development. Nat Genet. 2003;35:270–276. - PubMed
-
- Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell. 2007;128:29–43. - PubMed
-
- Ballif BA, Arnaud L, Arthur WT, Guris D, Imamoto A, Cooper JA. Activation of a Dab1/CrkL/C3G/Rap1 pathway in Reelin-stimulated neurons. Curr Biol. 2004;14:606–610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
