Behavioral and cerebellar transmission deficits in mice lacking the autism-linked gene islet brain-2
- PMID: 21048139
- PMCID: PMC3200367
- DOI: 10.1523/JNEUROSCI.1161-10.2010
Behavioral and cerebellar transmission deficits in mice lacking the autism-linked gene islet brain-2
Abstract
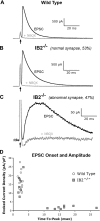
Deletion of the human SHANK3 gene near the terminus of chromosome 22q is associated with Phelan-McDermid syndrome and autism spectrum disorders. Nearly all such deletions also span the tightly linked IB2 gene. We show here that IB2 protein is broadly expressed in the brain and is highly enriched within postsynaptic densities. Experimental disruption of the IB2 gene in mice reduces AMPA and enhances NMDA receptor-mediated glutamatergic transmission in cerebellum, changes the morphology of Purkinje cell dendritic arbors, and induces motor and cognitive deficits suggesting an autism phenotype. These findings support a role for human IB2 mutation as a contributing genetic factor in Chr22qter-associated cognitive disorders.
Figures







References
-
- Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci. 2005;23:183–187. - PubMed
-
- Bobée S, Mariette E, Tremblay-Leveau H, Caston J. Effects of early midline cerebellar lesion on cognitive and emotional functions in the rat. Behav Brain Res. 2000;112:107–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
