Recent thymic emigrants are biased against the T-helper type 1 and toward the T-helper type 2 effector lineage
- PMID: 21048154
- PMCID: PMC3056472
- DOI: 10.1182/blood-2010-07-299263
Recent thymic emigrants are biased against the T-helper type 1 and toward the T-helper type 2 effector lineage
Abstract
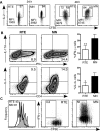
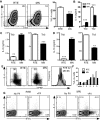
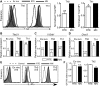
After intrathymic development, T cells exit the thymus and join the peripheral T-cell pool. Such recent thymic emigrants (RTEs) undergo both phenotypic and functional maturation during the first 3 weeks they reside in the periphery. Using a well-controlled in vitro polarization scheme, we now show that CD4(+) RTEs are defective in T-helper (Th) type 0 (Th0), Th1, Th17, and regulatory T-cell lineage commitment, with dampened cytokine production and transcription factor expression. In contrast, CD4(+) RTES are biased toward the Th2 lineage both in vitro and in vivo, with more robust interleukin-4, interleukin-5, and interleukin-13 production than their mature naive counterparts. Coculture experiments demonstrate that mature naive T cells influence neighboring RTEs in their Th responses. In adoptive hosts, CD4(+) RTEs drive production of the Th2-associated antibody isotype immunoglobulin G1 and mediate airway inflammatory disease. This bias in RTEs likely results from dampened negative regulation of the Th2 lineage by diminished levels of T-bet, a key Th1 transcription factor. CD4(+) RTEs thus represent a transitional population with a distinct interpretation of, and response to, immunologic cues. These characteristics may be beneficial during the postthymic maturation period by leading to the avoidance of inappropriate immune responses, particularly in lymphopenic neonates and adults.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

