Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease
- PMID: 21048217
- PMCID: PMC3170139
- DOI: 10.1126/scitranslmed.3001417
Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease
Abstract
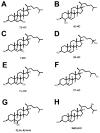
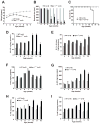
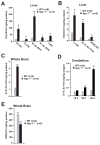
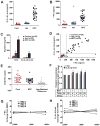
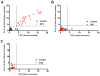
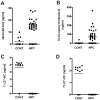
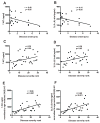
Niemann-Pick type C1 (NPC1) disease is a rare progressive neurodegenerative disorder characterized by accumulation of cholesterol in the endolysosomes. Previous studies implicating oxidative stress in NPC1 disease pathogenesis raised the possibility that nonenzymatic formation of cholesterol oxidation products could serve as disease biomarkers. We measured these metabolites in the plasma and tissues of the Npc1(-/-) mouse model and found several cholesterol oxidation products that were elevated in Npc1(-/-) mice, were detectable before the onset of symptoms, and were associated with disease progression. Nonenzymatically formed cholesterol oxidation products were similarly increased in the plasma of all human NPC1 subjects studied and delineated an oxysterol profile specific for NPC1 disease. This oxysterol profile also correlated with the age of disease onset and disease severity. We further show that the plasma oxysterol markers decreased in response to an established therapeutic intervention in the NPC1 feline model. These cholesterol oxidation products are robust blood-based biochemical markers for NPC1 disease that may prove transformative for diagnosis and treatment of this disorder, and as outcome measures to monitor response to therapy.
Figures

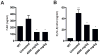
Comment in
-
Found in translation: a blood-based test for Niemann-Pick type C1.Biomark Med. 2011 Apr;5(2):201. doi: 10.2217/bmm.11.10. Biomark Med. 2011. PMID: 21473724 No abstract available.
References
-
- Vanier MT, Millat G. Clin Genet. 2003;64:269–281. - PubMed
-
- Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Strauss JF, 3rd, Ohno K, Zeigler M, Carmi R, Sokol J, Markie D, O’Neill RR, van Diggelen OP, Elleder M, Patterson MC, Brady RO, Vanier MT, Pentchev PG, Tagle DA. Science. 1997;277:228–231.
-
- Naureckiene S, Sleat DE, Lackland H, Fensom A, Vanier MT, Wattiaux R, Jadot M, Lobel P. Science. 2000;290:2298–2301. - PubMed
-
- Ory DS. Biochim Biophys Acta. 2000;1529:331–339. - PubMed
-
- Walkley SU, Suzuki K. Biochim Biophys Acta. 2004;1685:48–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

