Ex vivo-expanded human regulatory T cells prevent the rejection of skin allografts in a humanized mouse model
- PMID: 21048528
- PMCID: PMC3672995
- DOI: 10.1097/TP.0b013e3181ff8772
Ex vivo-expanded human regulatory T cells prevent the rejection of skin allografts in a humanized mouse model
Abstract
Background: Composite tissue transplantation effectively reconstructs the most complex defects, but its use is limited because of harmful immunosuppression and the high susceptibility of skin to rejection. Development of tolerance is an ideal solution, and protocols using regulatory T cells (Tregs) to achieve this have been promising in experimental animal models. The aim of this study was to investigate the ability of human Tregs to regulate immune responses to a human skin allograft in vivo.
Methods: We isolated and expanded naturally occurring CD127loCD25+CD4+ human Tregs from peripheral blood mononuclear cells (PBMCs) and examined their phenotype and suppressive activity in vitro. Using a clinically relevant chimeric humanized mouse system, we transplanted mice with human skin grafts followed by allogeneic populations of PBMCs with or without Tregs derived from the same PBMC donor.
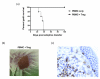
Results: Ex vivo-expanded Tregs maintain the appropriate Treg markers and retain suppressive activity against allostimulated and polyclonally stimulated autologous PBMCs in vitro. Mice receiving allogeneic PBMCs alone consistently reject human skin grafts, whereas those also receiving Tregs display stable long-term human skin transplant survival along with a reduction in the CD8+ human cellular graft infiltrate.
Conclusions: We show for the first time the unique ability of human Tregs to prevent the rejection of a skin allograft in vivo, highlighting the therapeutic potential of these cells clinically.
Figures





References
-
- Petruzzo P, Lanzetta M, Dubernard JM, et al. The international registry on hand and composite tissue transplantation. Transplantation. 2008;86(4):487. - PubMed
-
- Ravindra KV, Buell JF, Kaufman CL, et al. Hand transplantation in the United States: experience with 3 patients. Surgery. 2008;144(4):638. - PubMed
-
- Buttemeyer R, Jones NF, Min Z, Rao U. Rejection of the component tissues of limb allografts in rats immunosuppressed with FK-506 and cyclosporine. Plast Reconstr Surg. 1996;97(1):139. - PubMed
-
- Humar A, Hassoun A, Kandaswamy R, Payne WD, Sutherland DE, Matas AJ. Immunologic factors: the major risk for decreased long-term renal allograft survival. Transplantation. 1999;68(12):1842. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

