Integrating carbon-halogen bond formation into medicinal plant metabolism
- PMID: 21048708
- PMCID: PMC3058899
- DOI: 10.1038/nature09524
Integrating carbon-halogen bond formation into medicinal plant metabolism
Abstract
Halogenation, which was once considered a rare occurrence in nature, has now been observed in many natural product biosynthetic pathways. However, only a small fraction of halogenated compounds have been isolated from terrestrial plants. Given the impact that halogenation can have on the biological activity of natural products, we reasoned that the introduction of halides into medicinal plant metabolism would provide the opportunity to rationally bioengineer a broad variety of novel plant products with altered, and perhaps improved, pharmacological properties. Here we report that chlorination biosynthetic machinery from soil bacteria can be successfully introduced into the medicinal plant Catharanthus roseus (Madagascar periwinkle). These prokaryotic halogenases function within the context of the plant cell to generate chlorinated tryptophan, which is then shuttled into monoterpene indole alkaloid metabolism to yield chlorinated alkaloids. A new functional group-a halide-is thereby introduced into the complex metabolism of C. roseus, and is incorporated in a predictable and regioselective manner onto the plant alkaloid products. Medicinal plants, despite their genetic and developmental complexity, therefore seem to be a viable platform for synthetic biology efforts.
Figures


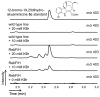
Comment in
-
Chemical biology: Synthetic metabolism goes green.Nature. 2010 Nov 18;468(7322):380-1. doi: 10.1038/468380a. Nature. 2010. PMID: 21085165 No abstract available.
References
-
- Neumann CS, Fujimori DG, Walsh CT. Halogenation strategies in natural products biosynthesis. Chem Biol. 2008;15:99–109. - PubMed
-
- Gribble GW. The diversity of naturally produced organohalogens. Chemosphere. 2003;52:289–297. - PubMed
-
- Vaillancourt FH, Yeh E, Vosburg DA, Garneau-Tsodiova S, Walsh CT. Nature’s inventory of halogenation catalysts: oxidative strategies predominate. Chem Rev. 2006;106:3364–3378. - PubMed
-
- van Peé KH, Patallo EP. Flavin-dependent halogenases involved in secondary metabolism in bacteria. Appl Microbiol Biotechnol. 2006;70:631–641. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

