Spatial variability in airborne bacterial communities across land-use types and their relationship to the bacterial communities of potential source environments
- PMID: 21048802
- PMCID: PMC3105744
- DOI: 10.1038/ismej.2010.167
Spatial variability in airborne bacterial communities across land-use types and their relationship to the bacterial communities of potential source environments
Abstract
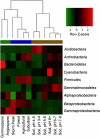
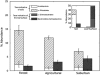
Although bacteria are ubiquitous in the near-surface atmosphere and they can have important effects on human health, airborne bacteria have received relatively little attention and their spatial dynamics remain poorly understood. Owing to differences in meteorological conditions and the potential sources of airborne bacteria, we would expect the atmosphere over different land-use types to harbor distinct bacterial communities. To test this hypothesis, we sampled the near-surface atmosphere above three distinct land-use types (agricultural fields, suburban areas and forests) across northern Colorado, USA, sampling five sites per land-use type. Microbial abundances were stable across land-use types, with ∼10(5)-10(6) bacterial cells per m(3) of air, but the concentrations of biological ice nuclei, determined using a droplet freezing assay, were on average two and eight times higher in samples from agricultural areas than in the other two land-use types. Likewise, the composition of the airborne bacterial communities, assessed via bar-coded pyrosequencing, was significantly related to land-use type and these differences were likely driven by shifts in the sources of bacteria to the atmosphere across the land-uses, not local meteorological conditions. A meta-analysis of previously published data shows that atmospheric bacterial communities differ from those in potential source environments (leaf surfaces and soils), and we demonstrate that we may be able to use this information to determine the relative inputs of bacteria from these source environments to the atmosphere. This work furthers our understanding of bacterial diversity in the atmosphere, the terrestrial controls on this diversity and potential approaches for source tracking of airborne bacteria.
Figures


 ) Total bacterial abundance in the collected samples from the Colorado Front Range as determined via direct microscopy, and right y axis, (□) total number of high-temperature ice nuclei as determined via the drop-freeze assay. Letters above the bars indicate a significant difference at P<0.05. Error bars indicate±1 s.e.m.
) Total bacterial abundance in the collected samples from the Colorado Front Range as determined via direct microscopy, and right y axis, (□) total number of high-temperature ice nuclei as determined via the drop-freeze assay. Letters above the bars indicate a significant difference at P<0.05. Error bars indicate±1 s.e.m.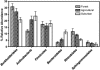

References
-
- Albrecht A, Witzenberger R, Bernzen U, Jackel U. Detection of airborne microbes in a composting facility by cultivation based and cultivation-independent methods. Ann Agric Environ Med. 2007;14:81–85. - PubMed
-
- Amato P, Ménager M, Sancelme M, Laj P, Mailhot G, Delort A-M. Microbial population in cloud water at the Puy de Dôme: Implications for the chemistry of clouds. Atmos Environ. 2005;39:4143–4153.
-
- Bauer H, Giebl H, Hitzenberger R, Kasper-Giebl A, Reischl G, Zibuschka F, et al. Airborne bacteria as cloud condensation nuclei. J Geophys Res. 2003;108:4658–4663.

