Acceleration of functional maturation and differentiation of neonatal porcine islet cell monolayers shortly in vitro cocultured with microencapsulated sertoli cells
- PMID: 21048849
- PMCID: PMC2956457
- DOI: 10.4061/2010/587213
Acceleration of functional maturation and differentiation of neonatal porcine islet cell monolayers shortly in vitro cocultured with microencapsulated sertoli cells
Abstract
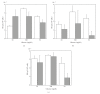
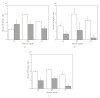
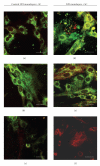
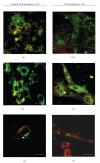
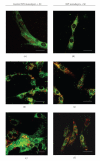
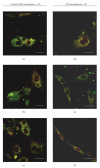
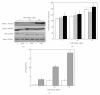
The limited availability of cadaveric human donor pancreata as well as the incomplete success of the Edmonton protocol for human islet allografts fasten search for new sources of insulin the producing cells for substitution cell therapy of insulin-dependent diabetes mellitus (T1DM). Starting from isolated neonatal porcine pancreatic islets (NPIs), we have obtained cell monolayers that were exposed to microencapsulated monolayered Sertoli cells (ESCs) for different time periods (7, 14, 21 days). To assess the development of the cocultured cell monolayers, we have studied either endocrine cell phenotype differentiation markers or c-kit, a hematopoietic stem cell marker, has recently been involved with growth and differentiation of β-cell subpopulations in human as well as rodent animal models. ESC which were found to either accelerate maturation and differentiation of the NPIs β-cell phenotype or identify an islet cell subpopulation that was marked positively for c-kit. The insulin/c-kit positive cells might represent a new, still unknown functionally immature β-cell like element in the porcine pancreas. Acceleration of maturation and differentiation of our NPI cell monolayers might generate a potential new opportunity to develop insulin-producing cells that may suite experimental trials for cell therapy of T1DM.
Figures

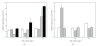
References
-
- Nathan DM. The rationale for glucose control in diabetes mellitus. Endocrinology and Metabolism Clinics of North America. 1992;21(2):221–235. - PubMed
-
- Shapiro AMJ, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. The New England Journal of Medicine. 2006;355(13):1318–1330. - PubMed
-
- Luca G, Nastruzzi C, Calvitti M, et al. Accelerated functional maturation of isolated neonatal porcine cell clusters: in vitro and in vivo post-transplant results in NOD mice. Cell Tranplantation. 2005;14:249–261. - PubMed
LinkOut - more resources
Full Text Sources

