Purification, Characterization, and Effect of Thiol Compounds on Activity of the Erwinia carotovora L-Asparaginase
- PMID: 21048860
- PMCID: PMC2956972
- DOI: 10.4061/2010/165878
Purification, Characterization, and Effect of Thiol Compounds on Activity of the Erwinia carotovora L-Asparaginase
Abstract
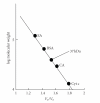
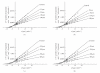
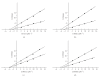
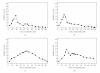
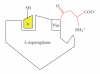
L-asparaginase was extracted from Erwinia carotovora and purified by ammonium sulfate fractionation (60-70%), Sephadex G-100, CM cellulose, and DEAE sephadex chromatography. The apparent Mr of enzyme under nondenaturing and denaturing conditions was 150 kDa and 37 ± 0.5 kDa, respectively. L-asparaginase activity was studied in presence of thiols, namely, L-cystine (Cys), L-methionine (Met), N-acetyl cysteine (NAC), and reduced glutathione (GSH). Kinetic parameters in presence of thiols (10-400 μM) showed an increase in V(max) values (2000, 2223, 2380, 2500, and control 1666.7 μmoles mg(-1)min(-1)) and a decrease in K(m) values (0.086, 0.076, 0.062, 0.055 and control 0.098 mM) indicating nonessential mode of activation. K(A) values displayed propensity to bind thiols. A decrease in V(max)/K(m) ratio in concentration plots showed inverse relationship between free thiol groups (NAC and GSH) and bound thiol group (Cys and Met). Enzyme activity was enhanced in presence of thiol protecting reagents like dithiothreitol (DTT), 2-mercaptoethanol (2-ME), and GSH, but inhibited by p-chloromercurybenzoate (PCMB) and iodoacetamide (IA).
Figures



References
-
- Borisova AA, Eldarov MA, Zhgun AA, et al. Purification and some properties of recombinant Erwinia carotovora L-asparaginase, expressed in E. coli cells. Biomeditsinskaya Khimiya. 2003;49(5):502–507. - PubMed
-
- North ACT, Wade HE, Cammack KA. Physicochemical studies of L-Asparaginase from Erwinia carotovora . Nature. 1969;224(5219):594–595. - PubMed
-
- Ammon HL, Murphy KC, Chandrasekhar K, Wlodawer A. Preliminary crystallographic study of an l-asparaginase from Vibrio succinogenes. Journal of Molecular Biology. 1985;184(1):179–181. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

