Enduring medial perforant path short-term synaptic depression at high pressure
- PMID: 21048901
- PMCID: PMC2967425
- DOI: 10.3389/fncel.2010.00128
Enduring medial perforant path short-term synaptic depression at high pressure
Abstract
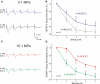
The high pressure neurological syndrome develops during deep-diving (>1.1 MPa) involving impairment of cognitive functions, alteration of synaptic transmission and increased excitability in cortico-hippocampal areas. The medial perforant path (MPP), connecting entorhinal cortex with the hippocampal formation, displays synaptic frequency-dependent-depression (FDD) under normal conditions. Synaptic FDD is essential for specific functions of various neuronal networks. We used rat cortico-hippocampal slices and computer simulations for studying the effects of pressure and its interaction with extracellular Ca(2+) ([Ca(2+)](o)) on FDD at the MPP synapses. At atmospheric pressure, high [Ca(2+)](o) (4-6 mM) saturated single MPP field EPSP (fEPSP) and increased FDD in response to short trains at 50 Hz. High pressure (HP; 10.1 MPa) depressed single fEPSPs by 50%. Increasing [Ca(2+)](o) to 4 mM at HP saturated synaptic response at a subnormal level (only 20% recovery of single fEPSPs), but generated a FDD similar to atmospheric pressure. Mathematical model analysis of the fractions of synaptic resources used by each fEPSP during trains (normalized to their maximum) and the total fraction utilized within a train indicate that HP depresses synaptic activity also by reducing synaptic resources. This data suggest that MPP synapses may be modulated, in addition to depression of single events, by reduction of synaptic resources and then may have the ability to conserve their dynamic properties under different conditions.
Keywords: HPNS; dentate gyrus; entorhinal cortex; granule cells; hippocampus; hyperbaric helium pressure; rat; synaptic dynamics.
Figures
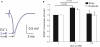
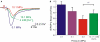
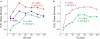
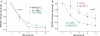
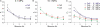
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

