Herpesvirus BACs: past, present, and future
- PMID: 21048927
- PMCID: PMC2965428
- DOI: 10.1155/2011/124595
Herpesvirus BACs: past, present, and future
Erratum in
-
Corrigendum to "Herpesvirus BACs: Past, Present, and Future".Biomed Res Int. 2019 Jul 8;2019:6870815. doi: 10.1155/2019/6870815. eCollection 2019. Biomed Res Int. 2019. PMID: 31360721 Free PMC article.
Abstract
The herpesviridae are a large family of DNA viruses with large and complicated genomes. Genetic manipulation and the generation of recombinant viruses have been extremely difficult. However, herpesvirus bacterial artificial chromosomes (BACs) that were developed approximately 10 years ago have become useful and powerful genetic tools for generating recombinant viruses to study the biology and pathogenesis of herpesviruses. For example, BAC-directed deletion mutants are commonly used to determine the function and essentiality of viral genes. In this paper, we discuss the creation of herpesvirus BACs, functional analyses of herpesvirus mutants, and future applications for studies of herpesviruses. We describe commonly used methods to create and mutate herpesvirus BACs (such as site-directed mutagenesis and transposon mutagenesis). We also evaluate the potential future uses of viral BACs, including vaccine development and gene therapy.
Figures

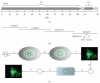
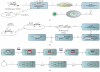
Similar articles
-
Recent advances in herpesvirus genetics using bacterial artificial chromosomes.Mol Genet Metab. 2001 Jan;72(1):8-14. doi: 10.1006/mgme.2000.3123. Mol Genet Metab. 2001. PMID: 11161823 Review.
-
Forward with BACs: new tools for herpesvirus genomics.Trends Genet. 2000 Jun;16(6):254-9. doi: 10.1016/s0168-9525(00)02015-1. Trends Genet. 2000. PMID: 10827452 Review.
-
Recent advances in cloning herpesviral genomes as infectious bacterial artificial chromosomes.Cell Cycle. 2011 Feb 1;10(3):434-40. doi: 10.4161/cc.10.3.14708. Epub 2011 Feb 1. Cell Cycle. 2011. PMID: 21245660 Free PMC article. Review.
-
Back to BAC: the use of infectious clone technologies for viral mutagenesis.Viruses. 2012 Feb;4(2):211-35. doi: 10.3390/v4020211. Epub 2012 Feb 3. Viruses. 2012. PMID: 22470833 Free PMC article. Review.
-
Direct cloning of a herpesvirus genome for rapid generation of infectious BAC clones.J Adv Res. 2023 Jan;43:97-107. doi: 10.1016/j.jare.2022.02.012. Epub 2022 Feb 23. J Adv Res. 2023. PMID: 36585118 Free PMC article.
Cited by
-
Targeted Mutagenesis of Guinea Pig Cytomegalovirus Using CRISPR/Cas9-Mediated Gene Editing.J Virol. 2016 Jul 11;90(15):6989-6998. doi: 10.1128/JVI.00139-16. Print 2016 Aug 1. J Virol. 2016. PMID: 27226370 Free PMC article.
-
Complete Genome Sequence of a Human Cytomegalovirus Strain AD169 Bacterial Artificial Chromosome Clone.Genome Announc. 2016 Mar 31;4(2):e00091-16. doi: 10.1128/genomeA.00091-16. Genome Announc. 2016. PMID: 27034483 Free PMC article.
-
One of the Triple Poly(A) Signals in the M112-113 Gene Is Important and Sufficient for Stabilizing the M112-113 mRNA and the Replication of Murine Cytomegalovirus.Viruses. 2020 Aug 28;12(9):954. doi: 10.3390/v12090954. Viruses. 2020. PMID: 32872150 Free PMC article.
-
An EBV recombinant deleted for residues 130-159 in EBNA3C can deregulate p53/Mdm2 and Cyclin D1/CDK6 which results in apoptosis and reduced cell proliferation.Oncotarget. 2016 Apr 5;7(14):18116-34. doi: 10.18632/oncotarget.7502. Oncotarget. 2016. PMID: 26908453 Free PMC article.
-
Rapid and efficient in vitro excision of BAC sequences from herpesvirus genomes using Cre-mediated recombination.J Virol Methods. 2018 Nov;261:67-70. doi: 10.1016/j.jviromet.2018.08.006. Epub 2018 Aug 6. J Virol Methods. 2018. PMID: 30092252 Free PMC article.
References
-
- Murray PR, Rosenthal KS, Pfaller MA. Medical Microbiology. St. Louis, Mo, USA: Mosby; 2002.
-
- Pellett PE, Roizman B. The family ferpesviridae: a brief introduction. In: Knipe DM, Howley PM, editors. Field's Virology. 5th edition. Philadelphia, Pa, USA: Lippincott Williams & Wilkins; 2007.
-
- Roizman B, Knipe DM, Whitley R. Herpes simplex viruses. In: Knipe DM, Howley PM, editors. Field's Virology. 5th edition. Philadelphia, Pa, USA: Lippincott Williams & Wilkins; 2007.
-
- Cohen JI, Straus SE, Arvin A. Varicella-zoster virus replication, pathogenesis, and management. In: Knipe DM, Howley PM, editors. Field's Virology. 5th edition. Philadelphia, Pa, USA: Lippincott Williams & Wilkins; 2007.
-
- Mocarski ES, Shenk T, Pass RF. Cytomegaloviruses. In: Knipe DM, Howley PM, editors. Field's Virology. 5th edition. Philadelphia, Pa, USA: Lippincott Williams & Wilkins; 2007.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
