Dexamethasone treatment induces the reprogramming of pancreatic acinar cells to hepatocytes and ductal cells
- PMID: 21048969
- PMCID: PMC2965100
- DOI: 10.1371/journal.pone.0013650
Dexamethasone treatment induces the reprogramming of pancreatic acinar cells to hepatocytes and ductal cells
Erratum in
-
Correction: Dexamethasone Treatment Induces the Reprogramming of Pancreatic Acinar Cells to Hepatocytes and Ductal Cells.PLoS One. 2019 Jul 2;14(7):e0219419. doi: 10.1371/journal.pone.0219419. eCollection 2019. PLoS One. 2019. PMID: 31265474 Free PMC article.
Abstract
Background: The pancreatic exocrine cell line AR42J-B13 can be reprogrammed to hepatocytes following treatment with dexamethasone. The question arises whether dexamethasone also has the capacity to induce ductal cells as well as hepatocytes.
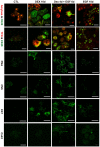
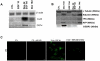
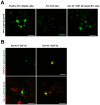
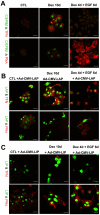
Methodology/principal findings: AR42J-B13 cells were treated with and without dexamethasone and analyzed for the expression of pancreatic exocrine, hepatocyte and ductal markers. Addition of dexamethasone inhibited pancreatic amylase expression, induced expression of the hepatocyte marker transferrin as well as markers typical of ductal cells: cytokeratin 7 and 19 and the lectin peanut agglutinin. However, the number of ductal cells was low compared to hepatocytes. The proportion of ductal cells was enhanced by culture with dexamethasone and epidermal growth factor (EGF). We established several features of the mechanism underlying the transdifferentiation of pancreatic exocrine cells to ductal cells. Using a CK19 promoter reporter, we show that a proportion of the ductal cells arise from differentiated pancreatic exocrine-like cells. We also examined whether C/EBPβ (a transcription factor important in the conversion of pancreatic cells to hepatocytes) could alter the conversion from acinar cells to a ductal phenotype. Overexpression of an activated form of C/EBPβ in dexamethasone/EGF-treated cells provoked the expression of hepatocyte markers and inhibited the expression of ductal markers. Conversely, ectopic expression of a dominant-negative form of C/EBPβ, liver inhibitory protein, inhibited hepatocyte formation in dexamethasone-treated cultures and enhanced the ductal phenotype.
Conclusions/significance: These results indicate that hepatocytes and ductal cells may be induced from pancreatic exocrine AR42J-B13 cells following treatment with dexamethasone. The conversion from pancreatic to hepatocyte or ductal cells is dependent upon the expression of C/EBPβ.
Conflict of interest statement
Figures



References
-
- Paner GP, Thompson KS, Reyes CV. Hepatoid carcinoma of the pancreas. Cancer. 2000;88:1582–1589. - PubMed
-
- Cingolani N, Shaco-Levy R, Farruggio A, Klimstra DS, Rosai J. Alpha-fetoprotein production by pancreatic tumors exhibiting acinar cell differentiation: study of five cases, one arising in a mediastinal teratoma. Hum Pathol. 2000;31:938–944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

