Influences of intermittent preventive treatment and persistent multiclonal Plasmodium falciparum infections on clinical malaria risk
- PMID: 21048970
- PMCID: PMC2965101
- DOI: 10.1371/journal.pone.0013649
Influences of intermittent preventive treatment and persistent multiclonal Plasmodium falciparum infections on clinical malaria risk
Abstract
Background: Intermittent preventive treatment (IPT) of malaria involves administration of curative doses of antimalarials at specified time points to vulnerable populations in endemic areas, regardless whether a subject is known to be infected. The effect of this new intervention on the development and maintenance of protective immunity needs further understanding. We have investigated how seasonal IPT affects the genetic diversity of Plasmodium falciparum infections and the risk of subsequent clinical malaria.
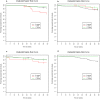
Material and methods: The study included 2227 Ghanaian children (3-59 months) who were given sulphadoxine-pyrimethamine (SP) bimonthly, artesunate plus amodiaquine (AS+AQ) monthly or bimonthly, or placebo monthly for six months spanning the malaria transmission season. Blood samples collected at three post-interventional surveys were analysed by genotyping of the polymorphic merozoite surface protein 2 gene. Malaria morbidity and anaemia was monitored during 12 months follow-up.
Results: Monthly IPT with AS+AQ resulted in a marked reduction in number of concurrent clones and only children parasite negative just after the intervention period developed clinical malaria during follow-up. In the placebo group, children without parasites as well as those infected with ≥2 clones had a reduced risk of subsequent malaria. The bimonthly SP or AS+AQ groups had similar number of clones as placebo after intervention; however, diversity and parasite negativity did not predict the risk of malaria. An interaction effect showed that multiclonal infections were only associated with protection in children without intermittent treatment.
Conclusion: Molecular typing revealed effects of the intervention not detected by ordinary microscopy. Effective seasonal IPT temporarily reduced the prevalence and genetic diversity of P. falciparum infections. The reduced risk of malaria in children with multiclonal infections only seen in untreated children suggests that persistence of antigenically diverse P. falciparum infections is important for the maintenance of protective malaria immunity in high transmission settings.
Conflict of interest statement
Figures



References
-
- WHO 2008. World Malaria Report. Available: http://malaria.who.int/wmr2008/malaria2008.pdf. Accessed 13 December 2009.
-
- Gies S, Coulibaly SO, Ouattara FT, D'Alessandro U. Individual efficacy of intermittent preventive treatment with sulfadoxine-pyrimethamine in primi- and secundigravidae in rural Burkina Faso: impact on parasitaemia, anaemia and birth weight. Trop Med Int Health. 2009;14:174–182. - PubMed
-
- Aponte JJ, Schellenberg D, Egan A, Breckenridge A, Carneiro I, et al. Efficacy and safety of intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in African infants: a pooled analysis of six randomised, placebo-controlled trials. Lancet. 2009;374:1533–1542. - PubMed
-
- WHO 2009. Report of the Technical Consultation on Intermittent Preventive Treatment in Infants (IPTi), Technical Expert Group on Preventive Chemotherapy,23-24 April 2009 - WHO\HQ, Geneva, Switzerland. Document available at: http://www.who.int/malaria/publications/atoz/tegconsultiptiapr2009report....
-
- Cisse B, Sokhna C, Boulanger D, Milet J, Ba el H, et al. Seasonal intermittent preventive treatment with artesunate and sulfadoxine-pyrimethamine for prevention of malaria in Senegalese children: a randomised, placebo-controlled, double-blind trial. Lancet. 2006;367:659–667. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

