Oxytocin-Gly-Lys-Arg: a novel cardiomyogenic peptide
- PMID: 21048978
- PMCID: PMC2964328
- DOI: 10.1371/journal.pone.0013643
Oxytocin-Gly-Lys-Arg: a novel cardiomyogenic peptide
Abstract
Background: Oxytocin (OT), synthesized in the heart, has the ability to heal injured hearts and to promote cardiomyogenesis from stem cells. Recently, we reported that the OT-GKR molecule, a processing intermediate of OT, potently increased the spontaneous formation of cardiomyocytes (CM) in embryonic stem D3 cells and augmented glucose uptake in newborn rat CM above the level stimulated by OT. In the present experiments, we investigated whether OT-GKR exists in fetal and newborn rodent hearts, interacts with the OT receptors (OTR) and primes the generation of contracting cells expressing CM markers in P19 cells, a model for the study of early heart differentiation.
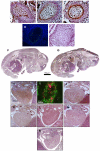
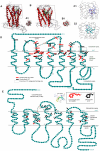
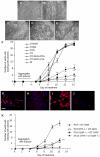
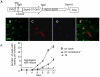
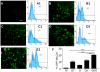
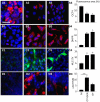
Methodology/principal findings: High performance liquid chromatography of newborn rat heart extracts indicated that OT-GKR was a dominant form of OT. Immunocytochemistry of mouse embryos (embryonic day 15) showed cardiac OT-GKR accumulation and OTR expression. Computerized molecular modeling revealed OT-GKR docking to active OTR sites and to V1a receptor of vasopressin. In embryonic P19 cells, OT-GKR induced contracting cell colonies and ventricular CM markers more potently than OT, an effect being suppressed by OT antagonists and OTR-specific small interfering (si) RNA. The V1a receptor antagonist and specific si-RNA also significantly reduced OT-GKR-stimulated P19 contracting cells. In comparison to OT, OT-GKR induced in P19 cells less α-actinin, myogenin and MyoD mRNA, skeletal muscle markers.
Conclusions/significance: These results raise the possibility that C-terminally extended OT molecules stimulate CM differentiation and contribute to heart growth during fetal life.
Conflict of interest statement
Figures

Similar articles
-
Oxytocin-Gly-Lys-Arg stimulates cardiomyogenesis by targeting cardiac side population cells.J Endocrinol. 2014 Jan 30;220(3):277-89. doi: 10.1530/JOE-13-0305. Print 2014 Mar. J Endocrinol. 2014. PMID: 24403294
-
Functional activity of the carboxyl-terminally extended oxytocin precursor Peptide during cardiac differentiation of embryonic stem cells.Stem Cells. 2008 Jan;26(1):45-54. doi: 10.1634/stemcells.2007-0289. Epub 2007 Oct 18. Stem Cells. 2008. PMID: 17951221
-
Oxytocin induces differentiation of P19 embryonic stem cells to cardiomyocytes.Proc Natl Acad Sci U S A. 2002 Jul 9;99(14):9550-5. doi: 10.1073/pnas.152302499. Epub 2002 Jul 1. Proc Natl Acad Sci U S A. 2002. PMID: 12093924 Free PMC article.
-
Oxytocin revisited: its role in cardiovascular regulation.J Neuroendocrinol. 2012 Apr;24(4):599-608. doi: 10.1111/j.1365-2826.2011.02235.x. J Neuroendocrinol. 2012. PMID: 21981277 Review.
-
Design of Oxytocin Analogs.Methods Mol Biol. 2019;2001:235-271. doi: 10.1007/978-1-4939-9504-2_11. Methods Mol Biol. 2019. PMID: 31134574 Review.
Cited by
-
U-shaped relation between plasma oxytocin levels and behavior in the trust game.PLoS One. 2012;7(12):e51095. doi: 10.1371/journal.pone.0051095. Epub 2012 Dec 5. PLoS One. 2012. PMID: 23227239 Free PMC article.
-
Protective cardiovascular and renal actions of vitamin D and estrogen.Front Biosci (Schol Ed). 2013 Jan 1;5(1):134-48. doi: 10.2741/s362. Front Biosci (Schol Ed). 2013. PMID: 23277041 Free PMC article. Review.
-
The Role of Oxytocin in Cardiovascular Protection.Front Psychol. 2020 Aug 25;11:2139. doi: 10.3389/fpsyg.2020.02139. eCollection 2020. Front Psychol. 2020. PMID: 32982875 Free PMC article. Review.
-
Oxytocin ameliorates the immediate myocardial injury in heart transplant through down regulation of the neutrophil dependent myocardial apoptosis.Heart Views. 2014 Apr;15(2):37-45. doi: 10.4103/1995-705X.137493. Heart Views. 2014. PMID: 25104981 Free PMC article.
-
Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics.J Neuroendocrinol. 2012 Apr;24(4):609-28. doi: 10.1111/j.1365-2826.2012.02303.x. J Neuroendocrinol. 2012. PMID: 22375852 Free PMC article. Review.
References
-
- Brownstein MJ, Russell JT, Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980;207:373–378. - PubMed
-
- Burbach JP, Luckman SM, Murphy D, Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol Rev. 2001;81:1197–1267. - PubMed
-
- Morris M, Castro M, Rose JC. Alterations in oxytocin prohormone processing during early development in the fetal sheep. Am J Physiol. 1992;263:R738–740. - PubMed
-
- Alstein M, Whitnall MH, House S, Key S, Gainer H. An immunochemical analysis of oxytocin and vasopressin prohormone processing in vivo. Peptides. 1988;9:87–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

