Glial innate immunity generated by non-aggregated alpha-synuclein in mouse: differences between wild-type and Parkinson's disease-linked mutants
- PMID: 21048992
- PMCID: PMC2964342
- DOI: 10.1371/journal.pone.0013481
Glial innate immunity generated by non-aggregated alpha-synuclein in mouse: differences between wild-type and Parkinson's disease-linked mutants
Abstract
Background: Parkinson's disease (PD) is a progressive neurodegenerative disorder characterized pathologically by the presence in the brain of intracellular protein inclusions highly enriched in aggregated alpha-synuclein (α-Syn). Although it has been established that progression of the disease is accompanied by sustained activation of microglia, the underlying molecules and factors involved in these immune-triggered mechanisms remain largely unexplored. Lately, accumulating evidence has shown the presence of extracellular α-Syn both in its aggregated and monomeric forms in cerebrospinal fluid and blood plasma. However, the effect of extracellular α-Syn on cellular activation and immune mediators, as well as the impact of familial PD-linked α-Syn mutants on this stimulation, are still largely unknown.
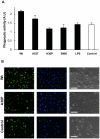
Methods and findings: In this work, we have compared the activation profiles of non-aggregated, extracellular wild-type and PD-linked mutant α-Syn variants on primary glial and microglial cell cultures. After stimulation of cells with α-Syn, we measured the release of Th1- and Th2- type cytokines as well as IP-10/CXCL10, RANTES/CCL5, MCP-1/CCL2 and MIP-1α/CCL3 chemokines. Contrary to what had been observed using cell lines or for the case of aggregated α-Syn, we found strong differences in the immune response generated by wild-type α-Syn and the familial PD mutants (A30P, E46K and A53T).
Conclusions: These findings might contribute to explain the differences in the onset and progression of this highly debilitating disease, which could be of value in the development of rational approaches towards effective control of immune responses that are associated with PD.
Conflict of interest statement
Figures
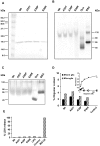

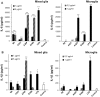
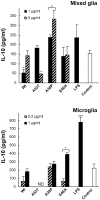
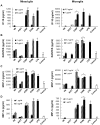
References
-
- Eriksen JL, Wszolek Z, Petrucelli L. Molecular pathogenesis of Parkinson disease. Arch Neurol. 2005;62:353–357. - PubMed
-
- Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. - PubMed
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

