Regulation of tumor immunity by tumor/dendritic cell fusions
- PMID: 21048993
- PMCID: PMC2964897
- DOI: 10.1155/2010/516768
Regulation of tumor immunity by tumor/dendritic cell fusions
Abstract
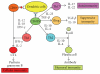
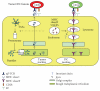
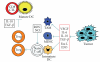
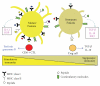
The goal of cancer vaccines is to induce antitumor immunity that ultimately will reduce tumor burden in tumor environment. Several strategies involving dendritic cells- (DCs)- based vaccine incorporating different tumor-associated antigens to induce antitumor immune responses against tumors have been tested in clinical trials worldwide. Although DCs-based vaccine such as fusions of whole tumor cells and DCs has been proven to be clinically safe and is efficient to enhance antitumor immune responses for inducing effective immune response and for breaking T-cell tolerance to tumor-associated antigens (TAAs), only a limited success has occurred in clinical trials. This paper reviews tumor immune escape and current strategies employed in the field of tumor/DC fusions vaccine aimed at enhancing activation of TAAs-specific cytotoxic T cells in tumor microenvironment.
Figures
References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. - PubMed
-
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annual Review of Immunology. 1991;9:271–296. - PubMed
-
- Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30(5):636–645. - PubMed
-
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

