The human host defense peptide LL-37 interacts with Neisseria meningitidis capsular polysaccharides and inhibits inflammatory mediators release
- PMID: 21049021
- PMCID: PMC2964311
- DOI: 10.1371/journal.pone.0013627
The human host defense peptide LL-37 interacts with Neisseria meningitidis capsular polysaccharides and inhibits inflammatory mediators release
Abstract
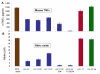
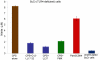
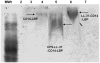
Capsular polysaccharides (CPS) are a major virulence factor in meningococcal infections and form the basis for serogroup designation and protective vaccines. Our work has identified meningococcal CPS as a pro-inflammatory ligand that functions through TLR2 and TLR4-MD2-dependent activation. We hypothesized that human cationic host defense peptides interact with CPS and influence its biologic activity. Accordingly, the interaction of meningococcal CPS with the human-derived cationic peptide LL-37, which is expressed by phagocytic and epithelial cells that interface with meningococci during infection, was investigated. LL-37 neutralized the pro-inflammatory activity of endotoxin-free CPS as assessed by TLR2 and TLR4-MD-2-dependent release of TNFα, IL-6 and IL-8 from human and murine macrophages. The cationic and hydrophobic properties of LL-37 were crucial for this inhibition, which was due to binding of LL-37 to CPS. LL-37 also inhibited the ability of meningococcal CPS to induce nitric oxide release, as well as TNFα and CXCL10 (IP-10) release from TLR4-sufficient and TLR4-deficient murine macrophages. Truncated LL-37 analogs, especially those that retained the antibacterial domain, inhibited vaccine grade CPS and meningococcal CPS prepared from the major serogroups (A, B C, Y and W135). Thus, LL-37 interaction with CPS was independent of specific glucan structure. We conclude that the capacity of meningococcal CPS to activate macrophages via TLR2 and TLR4-MD-2 can be inhibited by the human cationic host defense peptide LL-37 and propose that this impacts CPS-based vaccine responses.
Conflict of interest statement
Figures





References
-
- Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369:2196–2210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

